Variation of Orbital Energy & Effective Nuclear Charge with Atomic Number | Inorganic Chemistry PDF Download
Introduction
As the study in the field of chemistry furthered over the centuries, scientists preferred rationalizing the otherwise anomalous behavioral patterns of atoms and molecules, thus introducing the Periodic Table put forward by Mendeleev and the Atomic Structure by Ernest Rutherford, later corrected by Bohr. The idea of the atomic structure much like a solar model is flawed in many ways. Still, the perk here is it can give the fundamental understanding of the Nucleus posing as the Sun at the center and the Electrons orbiting the Nucleus matching planets with the disparity that the Electrons populate the regions of space, which are called the Orbitals and the orbitals have varied orbital energy levels.
As the magnitude of the changes increase, the magnitude of force also increases, and the forces decrease when the separation of charges is more. Therefore, the force of attraction between an electron and its nucleus is directly proportional to the distance between them. The electron is bound more tightly to the nucleus if the electron is closer to the nucleus. Hence, the electrons in the different shells which are at different distances have different energies.
Q.1. What is Principal Quantum Number (N)?
The foundation of orbitals chemistry starts with Bohr who established that electron orbitals represent an energy level in terms of their distance from the Nucleus. The Orbitals are named K, L, M, N… or 1, 2, 3, 4… in ascending order. These numbers are the Principal Quantum Numbers. A Principal Quantum number is denoted as 'n'. For example, for the K-orbital n = 1, for L-orbital n = 2, for M-orbital n = 3.
Q.2. What is the Azimuthal Quantum Number (L)?
Arnold Sommerfeld delved deeper into the orbitals chemistry, he viewed every orbital energy level or shell is made up of many subshells. He imagined that other than the circular orbits that Bohr established, there are elliptical orbits as well. The Azimuthal or Subsidiary quantum number helps to determine the ellipticity of the subshells. It is generally denoted as ‘l’.
To denote the value of 'l' instead of 1, 2, 3… some spectroscopic symbols are used -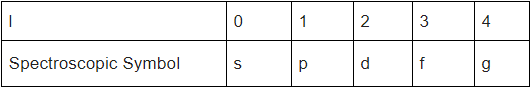 It should be noted that the subshells are energy levels as well, called Subsidiary orbital energy levels, so if we sort the subshells in ascending order in terms of their energy levels, it would be s < p < d < f.
It should be noted that the subshells are energy levels as well, called Subsidiary orbital energy levels, so if we sort the subshells in ascending order in terms of their energy levels, it would be s < p < d < f.
Orbital Energies
The energy of the electron in a hydrogen atom depends only on the principal quantum number, n.
The nucleus of a hydrogen atom has a charge of +1. If the electron is bound to a nucleus of arbitrary charge +Z, then the energy of the electron is
E = −RyZ2n2E = −RyZ2n2
Where, Ry is the Rydberg unit of energy where
1 Ry = 2.179877125595425×10−18×10−18J
= 13.60572374378387 eV
This equation is used for a single electron orbiting a single nucleus of charge +Z.
With the increase in quantum number, n increases (holding Z constant), and the energy increases i.e. it becomes less negative. In the limit that n goes to infinity then the energy goes to zero. With the increase in artitary charge, z, the energy decreases i.e. it becomes more negative.
A higher Z means a more positively charged nucleus, therefore it holds the electron tighter.
Calculating the Energy Level of an Orbital
In a single electron, Hydrogen-like atom, the orbital energy i.e. the energy of that one electron depends just on the principal quantum number (n). In orbitals chemistry when it comes to filling up the atom with electrons, the Aufbau principle tells the lower energy level orbitals always come first. The order followed here is 1s <2s < 2p < 3s <3p < 4s…
To easily memorize this anomalous behavior I strongly suggest following this diagram-
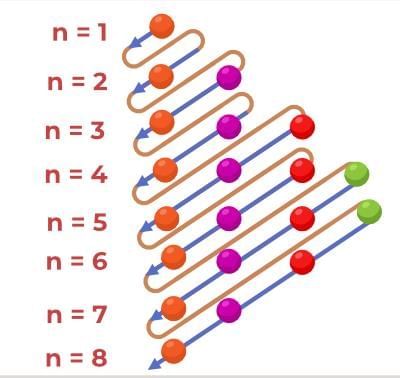
- Since the electrons are negatively charged particles, they repel each other. The stability of an atom depends on the attraction between the electrons, positively charged Nucleus and repulsive force within the electrons. The particle can only be stable if the total attractive interaction is more than the whole repulsive interaction.
- As we go down the periodic table, the atomic number increases and another factor comes into play here, i.e., shielding. Due to the presence of electrons in the inner shells, the total positive charge exerted by the Nucleus (Ze) is slightly hindered for the electrons in outer shells. The net positive charge felt by the electrons in the outer shells are termed as an effective nuclear charge (Zeffe ).
- The closer the orbital is to the Nucleus more tightly bound it would be. So an s-orbital electron will be more tightly bound to the Nucleus of that atom than a p-orbital.
- The s-orbital particles will be of a lesser charge as it has a lower orbital energy, which means it would be a more negative charge than the electrons in the p-orbital, which will have smaller energy for its higher orbital energy compared to the d-orbital electrons.
- In some cases, two orbitals may have the same n+l value; in those instances, the orbital with a lower n (principal quantum number) count will have low energy.
- In some cases two orbitals may have the same n+l value; in those instances the orbital with a lower n (principal quantum number) will have a low energy.
Q.3. Which Factors Affect Orbital Energy?
The s orbital electron is more tightly bound to the nucleus in comparison to the p orbital electron, which is more tightly bound with respect to a d orbital electron for a given value of the principal quantum number.
The orbital energy decreases in the same subshell with the increase in the atomic number (Zeff).
As compared to p orbital electrons, s orbital electrons have lesser amounts of energy and are more negative. In this case, the p orbital electrons will have lesser energy than that of d orbital electrons.
The extent of shielding from the nucleus is different for the electrons in different orbitals. Hence, it leads to the splitting of energy levels that have the same principal quantum number. Hence, the orbital energy depends on the values of both the principal quantum number and azimuthal quantum number, which are symbolized as n and l respectively. Therefore, the lower the value of (n + 1) for an orbital, the lower is its energy.
The Energy of Orbital in Hydrogen Atom
The energy of an electron in a hydrogen atom is calculated solely by the principal quantum, m (n). Therefore, the energy of the orbitals in hydrogen atom increases as follows :
1s < 2s = 2p < 3s = 3p = 3d <4s = 4p = 4d = 4f <..
The shapes of 2s and 2p orbitals are different, an electron has the same energy when it is in the 2s orbital as when it is present in a 2p orbital. The orbitals which have the same energy are called degenerate orbitals, whereas the orbitals which have the same energy are called degenerate orbitals.
The 1s orbital in a hydrogen atom is the most stable condition and is called the ground state and an electron living in this orbital is most strongly sustained by the nucleus. An electron in the 2s, 2p or higher orbitals in a hydrogen atom is in an excited state.
Q.4. Which of these orbitals has a lower orbital energy level 3d or 4s?
The n + l value of 3d orbital is (3 + 2) = 5, Similarly the (n + l) value of 4s is (4 + 0) = 4.
So the 3d orbital has a higher (n+l) value, thus has a higher orbital energy level.
4s
Q.5. Which of these orbitals has a higher orbital energy level 3d or 4p?
The (n+l) value of 3d orbital is (3+2) = 5, and 4p orbital is (4+1)=5. Both have the same (n+l) value with 3d having a lower n-count; thus, it is weaker and has a lower orbital energy level.
3d
Fun Facts
The letters s, p, d ,f represent the shape of the orbitals. The s-orbital is spherical, and the Nucleus is in its center. The p-orbital has a form of a pair of lobes on each side of the Nucleus, somewhat has a dumbbell kind of structure.
|
50 videos|92 docs|41 tests
|
FAQs on Variation of Orbital Energy & Effective Nuclear Charge with Atomic Number - Inorganic Chemistry
| 1. How does orbital energy vary with atomic number? |  |
| 2. What is the relationship between effective nuclear charge and atomic number? |  |
| 3. How does the variation of orbital energy with atomic number affect chemical properties? |  |
| 4. What is the significance of understanding the variation of orbital energy and effective nuclear charge? |  |
| 5. How does the variation of orbital energy and effective nuclear charge affect the atomic radius? |  |
















