Water Treatment | Civil Engineering SSC JE (Technical) - Civil Engineering (CE) PDF Download
Basic unit processes and operations for water treatment:
1. Screening
2. Plain sedimentation
3. Sedimentation with coagulation
4. Filtration
5. Disinfection
6. Aeration
7. Softening
8. Miscellaneous processes like fluoridation, de-salination, Re-carbonation and liming.
Screening:
- To remove large size particles like debris, trees, animals etc. There are two type of screens called coarse and fine screens.
Plain sedimentation:
- If the water contains suspended impurities of large size, it is very economical to remove them by preliminary sedimentation.
- The suspended impurities make the water-turbid; therefore, when they will be removed more uniform water will be available for the further treatment processes.
- Plain sedimentation is the process of removing suspended matters from the water by keeping it quiescent in tanks, so that suspended matter may settle down in the bottom due to force of gravity.
Sedimentation with coagulation:
- Purpose: This process enhances the removal of fine suspended particles that may not settle well through plain sedimentation. Coagulants like alum or ferric chloride are added to help particles clump together.
- Process: Coagulants are mixed with the raw water, forming flocs as they react with suspended particles. These flocs are then allowed to settle in a sedimentation basin, making it easier to remove them.
Filtration:
- Purpose: Filtration further refines water by removing smaller suspended particles, bacteria, and other microorganisms. It improves water clarity and quality.
- Process: Water passes through a bed of granular media (e.g., sand, anthracite coal) or a membrane, trapping particles and microorganisms. The filtered water is collected for further treatment.
Disinfection:
- Purpose: Disinfection is crucial for killing or inactivating harmful pathogens such as bacteria, viruses, and parasites to make the water safe for consumption.
- Process: Common disinfection methods include chlorination, UV (ultraviolet) irradiation, and ozonation. These methods destroy or deactivate microorganisms, ensuring safe drinking water.
Aeration:
- Purpose: Aeration is used to remove dissolved gases, especially excess dissolved iron and manganese, and to improve the taste and odor of water.
- Process: Air is introduced into water through diffusers or mechanical processes. As water comes into contact with the air, dissolved gases are released into the atmosphere.
Softening:
- Purpose: Water hardness, caused by high levels of calcium and magnesium ions, can lead to scaling in pipes and appliances. Softening reduces water hardness.
- Process: Softening typically involves the use of ion exchange resin columns. Calcium and magnesium ions are exchanged for sodium ions, resulting in softened water.
Miscellaneous Processes:
- Fluoridation: The addition of fluoride to water to promote dental health.
- Desalination: The removal of salt and other minerals to make seawater or brackish water suitable for drinking or industrial use.
- Re-carbonation: The adjustment of pH and alkalinity by adding carbon dioxide to water.
- Liming: The addition of lime (calcium hydroxide) to adjust pH and reduce acidity in water.
Factors influencing sedimentation:
(a) Velocity: Greater the flow area, lesser the velocity and hence more easily the particle will settle down.
(b) Viscosity: It is inversely proportional to temperature and settling velocity is inversely proportional to viscosity.
(c) Size, shape and specific gravity: Small size particles settle slowly. Settling velocity is directly proportional to specific gravity. Spherical particles settle fast because of its lesser surface area.
Stokes law:
- Any particle which does not alter its size, shape and weight while rising or settling in any fluid is called discrete particle.
- All the particles having more specific gravity than the liquid, will move vertically downward due to gravitational force.
- When any discrete particle is falling through a quiescent fluid, it will accelerate until the frictional resistance or drag force becomes equal to the gravitational forces acting upon the particle. At such stage the particle will settle at uniform velocity.
- This uniform velocity is called ‘Settling Velocity’ and is a very important factor.
The impelling force at uniform settling velocity is equal to the effective weight of the particle in fluid.
(a)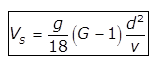 for d<0.1mm
for d<0.1mm
where,
Vs= velocity of settlement of particle in m/s
d= diametre of particle in m
G= specific gravity of particle
ν= Kinematic viscosity of water in m2/sec
(b)
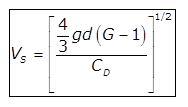
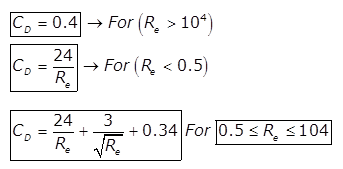
(c)
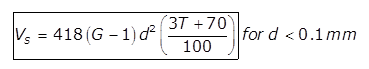
where,
T= Temperature of water in degree celsius
Vs in mm/sec
d is in mm
(e)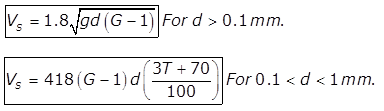
Type of sedimentation tanks:
(i) Horizontal flow: (a) Rectangular (b) Circular
(ii) Vertical flow
If Vs > V0 then only settling happens.
Horizontal sedimentation tank:
Theoretically depth does not have an influence on efficiency of tank.
Usually H = 3 to 4.5 m.
It is nothing but the overflow rate.
Over flow rate or surface over flow rate or surface loading can be said to be representing the settling velocity of the slowest settling particles,which are 100% removed.
Efficiency of sedimentation tank:
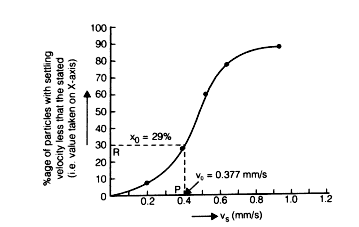
% of particle removed
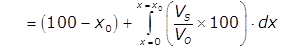
where xo corresponds to vo
Detention period: Average time for which water is detained in tank.
Circular tank:
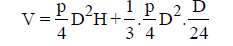
= D2[0.785H+0.011H]
Displacement efficiency = Flow through period / Detention period
=0.25 to 0.5.
Sludge removal must be done frequently
Sedimentation aided with coagulation:
By adding coagulants to water flow will form very fine colloidal particles. The very fine colloidal particles present in water get attracted and absorbed on these flocs forming the bigger sized flocculated particles.
Coagulation is a chemical technique which is directed towards the destabilization of the charged colloidal particles. Flocculation on other hand is the slow mixing technique which promotes the agglomeration of the destabilized particles. The entire process (coagulation) addition of particles and mixing (flocculation) is usually referred to as coagulation.
Chemicals used in coagulation are alum and iron salts like ferrous sulphate, ferric chloride. These chemicals are most effective when water is alkaline.
In absence of alkalinity add sodium carbonate or lime to increase alkalinity.
1. Alum: Al2 (SO4)3 18H2O
(i) Al2 (SO4)3 18H2O + 3 Ca(HCO3)2 → 3Ca SO4 + 2Al(OH)3 + 6CO2 + 18H2O
(ii) Al2 (SO4)3 18H2O + 3 Ca(OH)2 → 3Ca SO4 + 2Al(OH)3 + 18H2O
(iii) Al2 (SO4)3 18H2O + 3 Na2 CO3 → 3Na2 SO4 + 2Al(OH)3 + 3CO2 + 15H2O
2. Copperas [Ferrous sulphate] Fe SO4.7H2O
Copperas is generally added to raw water in conjunction with line. Lime may be added either to copperas or vice versa.
When lime is added first:
1. FeSO4.7H2O+Ca(OH)2 → CaSO4 + Fe (OH)2 + 7H2O
When copperas is added earlier to lime:
2. FeSO4.7H2O+Ca(HCO3)2 → Fe (HCO3)2 + CaSO4 + 7H2O
And
Fe (HCO3)2 + 2Ca (OH)2 → Fe (OH)2 + 2CaCO3 + 2H2O
The ferrous hydroxide formed in either case, further gets oxidized, forming ferric hydroxide.
4Fe (OH)2 + O2 + 2H2O → 4Fe (OH)3¯
3. Chlorinated copperas:
6 (FeSO4 7H2O) + 3Cl2 → 2Fe2(SO4)3 + 2FeCl3 + 42 H2O
the resultant combination of ferric sulphate and ferric chloride is known as chlorinated copperas.
Fe2 (SO4)3 + 3Ca (OH)2 → 3CaSO4 + 2Fe (OH)3
2FeCI3 + 3Ca (OH)2 → 3CaCI2+2Fe(OH)3
It is very effective coagulant for treating low pH waters.
4. Sodium aluminates (Na2AL2O4)
Na2Al2O4+ Ca (HCO3)2 → CaAl2O4 + Na2CO3 + CO2 + H2O
Na2Al2O4 + CaCl2 → l2O4 + 2 NaCI
Na2Al2O4 + CaSO4 → CaAl2O4 + Na2SO4
The constituents of a coagulation sedimentation tank:
1. Feeding device:
a) Dry feeding
b) wet feeding.
2. Mixing device :
a) Mixing basin with baffle wall.
b) Mechanical mixing basin.
The power required in flash mixing is expressed in terms of temporal mean velocity gradient G.
G'=(P/hV)1/2
G' = velocity gradient
h =Dynamic viscosity
V = Channel Volume
P = Power
3. Flocculation tank.
4. Sedimentation tank
FILTRATION:
The resultant water after sedimentation will not be pure, and may contain some very fine suspended particles and bacteria in it. To remove or to reduce the remaining impurities still further, the water is filtered through the beds of fine granular material, such as sand, etc. The process of passing the water through the beds of such granular materials is known as Filtration. There are two kinds of filters:
1. Slow sand gravity filters
2. Rapid sand filters.
Theory of filtration:
1) Mechanical straining: Particles of size more than voids will be filtered out.
2) Flocculation and sedimentation: Voids acts as tiny tanks.
3) Biological metabolism: - The bacteria present in sand particles will consume algae and produce harmless substance. These products forms a layer called “schmutzdecke”. (Dirty layer) or (Dirty skin) which further helps in absorbing impurities.
4) Electrolytic changes: The charges of sand grain and impurities are opposite in nature. They get neutralized and the character of water will change. So sand needs to be replaced frequently.
Construction of slow sand filters:
1. Enclosure tank: Open water-tight rectangular tank made of concrete. The bed slope is 1 in 100 towards the central drain. The depths vary from 2.5 m to 3.5 m the plan area very 100 to 2000 sq.m.
2. Filter media: Sand layers about 90 to 110 cm in depth placed over gravel support. D10 Varies from 0.2 to 0.4 mm. Cu varies from 1.8 to 3.0 finer media kept at top and coarser at bottom.
3. Base material: It is gravel and it supports the sand. Generally three to four layers each of 15-20 cm depth are used. The coarsest gravel is used in the bottom most layers and the first gravel is used in the topmost layer. Size varies from 3 mm to 65 mm
4. Under drainage system: The gravel support is laid on the top of an under drainage system. It consists of a central drain and lateral drains.
5. Inlet and outlet arrangements: Inlet chamber is to not disturb the sand layers. In order to maintain a constant discharge through the filter, an adjustable telescopic tube is used.
Operation and clearing:
Telescopic tube should be adjusted to get uniform discharge, due to head loss changes frequently. Cleaning is done by back washing. The top surface is removed by 1.5 to 3 cm. after washing water will not be pure for 24 to 36 hrs. To form schmutzdecke minimum time 2 to 3 days is required. Cleaning interval: 1 to 3 months.
Rate of filtration: 100-200 lit/hr/m .
Efficiency: Bacteria removal: 98-99%.
Removes all odours and tastes due to organic matter.
Turbidity can be removed only up to 500mg/l.
Rapid sand filters:
Filter media: D10 varies from 0.35 to 0.55 and Cu varies from 1.3 to 1.7. Thickness is about 60 to 90 cm depth.
Base material: 60 to 90 cm thick gravel of different sizes gravel size various from 3 mm to 40 mm.
Rate of filtration: - 3000 to 6000 lit/hr/m .
Efficiency: Bacterial removal: 80 to 90%.
Turbidity removal: 35 to 40 mg/l.
Slow sand filters Vs Rapid sand filters:
Base material: In SSF it varies from 3 to 65 mm in size and 30 to 75 cm in depth while in RSF it varies from 3 to 40 mm in size and its depth is slightly more, i.e. about 60 to 90 cm.
Filter sand: In SSF the effective size ranges between 0.2 to 0.4 mm and uniformity coefficient between 1.8 to 2.5 or 3.0. In RSF the effective size ranges between 0.35 to 0.55 and uniformity coefficient between 1.2 to 1.8.
Rate of filtration: In SSF it is small, such as 100 to 200 L/h/sq.m. of filter area while in RSF it is large, such as 3000 to 6000 L/h/sq.m. of filter area.
Flexibility: SSF are not flexible for meeting variation in demand whereas RSF are quite flexible for meeting reasonable variations in demand.
Post treatment required: Almost pure water is obtained from SSF. However, water may be disinfected slightly to make it completely safe. Disinfection is a must after RSF.
Method of cleaning: Scrapping and removing of the top 1.5 to 3 cm thick layer is done to clean SSF. To clean RSF, sand is agitated and backwashed with or without compressed air.
Loss of head: In case of SSF approx. 10 cm is the initial loss, and 0.8 to 1.2m is the final limit when cleaning is required. For RSF 0.3m is the initial loss, and 2.5 to 3.5m is the final limit when cleaning is required.
Disinfection:
The process of killing bacteria in filtered water is known as disinfection.
Minor methods:
a) Boiling
b) Treatment with lime
c) Treatment with zero
d) Treatment with iodine and bromine
e) Treatment with U.V.rays
f) Treatment with potassium permanganate
g) Treatment with silver called Electro-katadyn process
Chlorination: The process of killing bacteria in water by adding chlorine is called chlorination.
Disinfection action of chlorine:
Cl2+ H2O → HOCl ¯ + HCl
The hypochlorous acid is unstable and may break into hydrogen ions and hypochlorite ions.
Free available chlorine: The sum of hypochlorous acid and hypochlorite ions and molecular chlorine. Out of all forms of free available chlorine, the hypochlorous acid is the most destructive, being about 80 times more effective than the hypochlorous ions. For this reason pH value of water during chlorination is generally maintained less than 7.
The chlorine reacts with ammonia present in water.
NH3 + HOCl → NH2Cl + H2O Monochloramine (PH > 7.5)
NH3Cl + HOCl → NHCl2 + H2O Di - chloramine (PH 5-6.5)
NH3 Cl2 + HOCl → NCl3 + H2O Nitrogen trichloramine (PH < 4.0)
The chloramine so formed are stable and are found to possess disinfecting properties. They can also remove odour from water but only to a certain extent.
Free chlorine: -
When the added chlorine has consumed all the ammonia available in water then it would present as free chlorine.
Combined chlorine: -
The chlorine with ammonia in the form of chloramines is called the combined chlorine and is much less effective in causing disinfection compared to the free chlorine, being about 25 times less effective.
The free chlorine as well as combined chlorine will cause germicidal action on bacteria and pathogens. The free chlorine will instantaneously kill the pathogens, while the combined chlorine will provide long term germicidal effect.
Chlorine demand:
The chlorine consumed in oxidation of organic matter and in formation of chloramines is called chlorine demand of water. When once it gets satisfied, the chlorine will appear as a free chlorine or residual chlorine.
Various forms in which chlorine can be applied:
1. Liquid chlorine
2. Bleaching powder
3. Chlorine tablets
4. Chlorine di-oxide
5. Chloramines.
Bleaching powder : Ca (OCl)2
Type of chlorination:
1. Plain chlorination: - This term is used to indicate that only the chlorine treatment and no other treatment has been given to the raw water. Dose: 0.5 mg/lit.
2. Pre-chlorination: It is the process of applying chlorine to the water before filtration or even before sedimentation.
3. Past chlorination: This also called simply chlorination is the normal standard process of applying chlorine in the ends when all other treatments have been completed.
4. Double chlorination: The pre-chlorination and post chlorination are generally used in double chlorination.
5. Break point chlorination:
The point C is the point beyond which any further addition of chlorine will appear equally as free chlorine.
This point is called the break point. The addition of chlorine beyond break point is called break point chlorination.
The drop from B to C is due to inorganic matter. The deviation from 4 in AB portion is due to bacterial killing.
6. Super chlorination it indicates the addition of excessive amount of chlorine to water. This may be required in special cases of highly polluted water or during epidemics of water diseases.
7. De-chlorination: removing chlorine form water is called De-chlorination.
Testing of chlorine residuals:
1. Orthotolodine test
2. D.P.D test
3. Chlorotex test
4. Starch iodine test.
1. Orthotolodine test: In this test 10 ml of chlorinated sample of water is taken after the required contact period in a glass tube. To this add 0.1 ml of Orthotolodine solution. The color formed is noted after 5 sec and 5 min. Comparing this yellow color with standard colors gives free chlorine and combined chlorine available in water.
2. D.P.D test: In the test, palin’s DPD reagent is used. (Diethyl-Phenylene- Diamine). Compare the red color with standard color.
3. Chlorotex test: Chlorotex reagent is used. The immediate development of a color will indicate the presence of chlorine. Based on color concentration is decided.
4. Starch – iodine test : Potassium iodine is used. By adding this blue color will form. To remove blue color, add sodium thiosulphate and calculate chlorine.
WATER SOFTENING: -
1. To remove temporary hardness.
a) Boiling.
b) Addition of lime.
2. Permanent hardness:
a) Lime – soda process.
b) Zeolite or Base exchange or cation-exchange process
c) Demineralisaton process
|
2 videos|122 docs|55 tests
|
FAQs on Water Treatment - Civil Engineering SSC JE (Technical) - Civil Engineering (CE)
| 1. What is water treatment? |  |
| 2. What are the common methods used in water treatment? |  |
| 3. Why is water treatment necessary? |  |
| 4. How does water treatment impact the environment? |  |
| 5. Are there any alternative methods for water treatment? |  |


















