Worksheet: Chemical Changes and Reactions | Year 8 Chemistry (Cambridge) - Class 8 PDF Download
| Table of contents |

|
| Multiple Choice Questions (MCQs): |

|
| Fill in the Blanks: |

|
| True or False: |

|
| Match The Following |

|
Multiple Choice Questions (MCQs):
Q1. What type of substance is magnesium oxide?
A) An element
B) A mixture
C) A compound
D) A solution
Answer: C) A compound
Explanation: Magnesium oxide is a compound formed from the elements magnesium and oxygen. It has different properties from its constituent elements.
Q2. Which test confirms the presence of hydrogen gas?
A) Glowing splint test
B) Limewater test
C) Pop sound test
D) Universal indicator test
Answer: C) Pop sound test
Explanation: Hydrogen gas is confirmed by the 'pop' sound produced when a burning splint is held near it.
Q3. What happens in a precipitation reaction?
A) Soluble substances become gases.
B) Insoluble substances dissolve in water.
C) Soluble reactants form an insoluble product.
D) All reactants and products remain soluble.
Answer: C) Soluble reactants form an insoluble product.
Explanation: In a precipitation reaction, soluble reactants combine and form an insoluble substance, known as a precipitate, which makes the solution cloudy.
Q4. What indicates a chemical reaction has occurred?
A) Decrease in temperature only
B) Change in state only
C) Release of energy (heat or light), change in color, or formation of a precipitate
D) Sound production only
Answer: C) Release of energy (heat or light), change in color, or formation of a precipitate
Explanation: These are common indicators of chemical reactions, showing physical and chemical changes in the substances involved.
Q5. Which of the following is not a property of a solution?
A) Colored
B) Cloudy
C) Transparent
D) Homogeneous
Answer: B) Cloudy
Explanation: Solutions are homogeneous and transparent, meaning the particles are too small to see and evenly distributed, unlike cloudy mixtures where undissolved particles are visible.
Fill in the Blanks:
Q1. Magnesium burns in air to form ________ oxide.
Answer: Magnesium
Explanation: When magnesium burns, it reacts with oxygen in the air to form magnesium oxide.
Q2. A ________ splint relights in the presence of oxygen.
Answer: Glowing
Explanation: A glowing splint is used to test for oxygen; it will relight if oxygen is present due to the support of combustion.
Q3. ________ substances dissolve in water to form a clear solution.
Answer: Soluble
Explanation: Soluble substances dissolve completely in water, resulting in a solution that is clear and transparent.
Q4. The law of conservation of mass states that atoms are neither created nor ________.
Answer: Destroyed
Explanation: According to this law, atoms are only rearranged in chemical reactions, not created or destroyed.
Q5. The color change of a universal indicator can estimate the ________ of a solution.
Answer: pH
Explanation: Universal indicator changes color based on the acidity or alkalinity of a solution, providing a visual method for estimating pH.
True or False:
Q1. Copper sulfate solution is colorless.
Answer: False
Explanation: Copper sulfate solution is actually blue in color.
Q2. Atoms are rearranged in a chemical reaction.
Answer: True
Explanation: Chemical reactions involve the rearrangement of atoms from reactants to products, following the law of conservation of mass.
Q3. A solution can be cloudy if it contains undissolved particles.
Answer: True
Explanation: Cloudiness in a solution indicates the presence of undissolved particles, distinguishing it from a true solution.
Q4. Zinc reacts with copper sulfate to produce a gas.
Answer: False
Explanation: The reaction between zinc and copper sulfate primarily results in a color change due to the formation of zinc sulfate and copper metal; no gas is produced.
Q5. Neutralization results in the formation of water and possibly another soluble substance.
Answer: True
Explanation: Neutralization between an acid and a base typically results in water and sometimes a salt, which may dissolve in the solution.
Match The Following
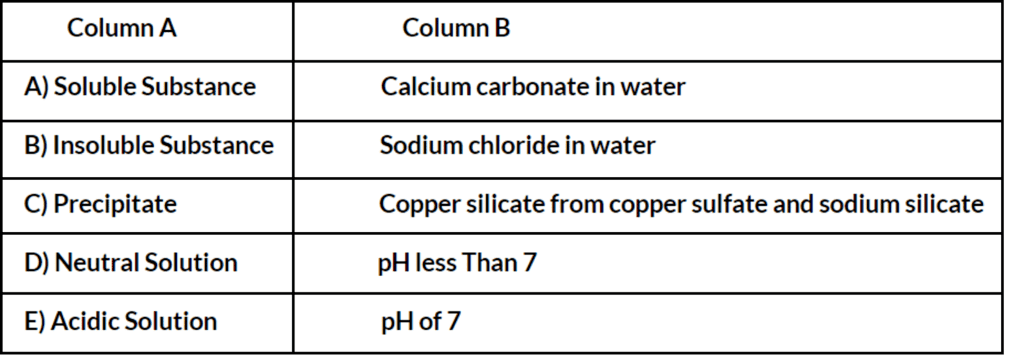
Answer:
A) Soluble Substance - Sodium chloride in water
B) Insoluble Substance - Calcium carbonate in water
C) Precipitate - Copper silicate from copper sulfate and sodium silicate
D) Neutral Solution - pH of 7
E) Acidic Solution - pH less than 7
Explanation: Each pair is matched based on the definitions of soluble and insoluble substances, what a precipitate is, and the characteristics of a neutral solution in terms of pH.
|
6 videos|14 docs|4 tests
|
FAQs on Worksheet: Chemical Changes and Reactions - Year 8 Chemistry (Cambridge) - Class 8
| 1. What are chemical changes? |  |
| 2. What are some examples of chemical reactions? |  |
| 3. How can you tell if a chemical change has occurred? |  |
| 4. What is the difference between physical and chemical changes? |  |
| 5. Why is it important to understand chemical changes and reactions? |  |














