Worksheet Solutions: Electricity: Magnetic and Heating Effects | Worksheets with Solutions for Class 8 PDF Download
| Table of contents |

|
| Multiple Choice Questions (MCQs) |

|
| Fill in the Blanks |

|
| True or False |

|
| Very Short Answer Questions (1 line) |

|
| Short Answer Questions |

|
| Match the Following |

|
Multiple Choice Questions (MCQs)
Instruction: Select the correct option for each question.
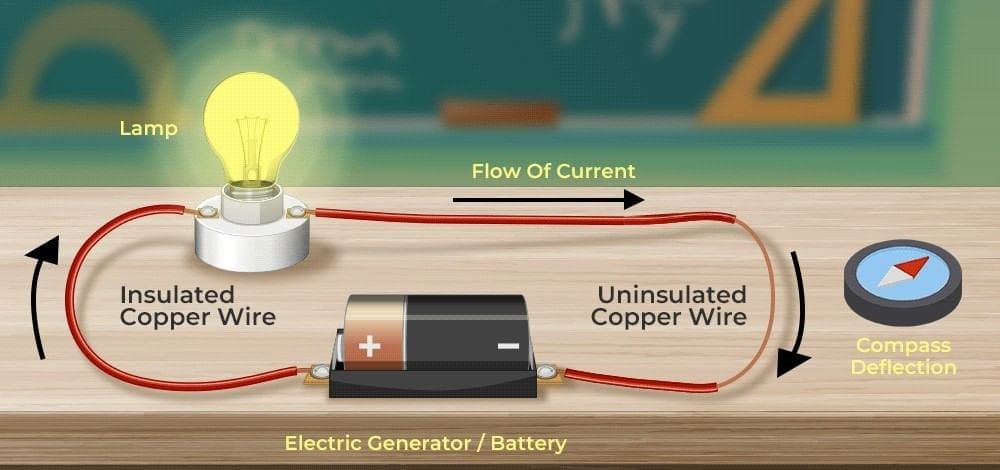
Q1. What did Oersted discover in 1820?
a) Heating effect of current
b) Electric charge of Earth
c) Magnetic effect of electric current
d) Structure of dry cell
Ans: c) Magnetic effect of electric current
Oersted observed that a current-carrying wire deflects a compass needle, proving current creates a magnetic field.
Q2. Which instrument can detect the magnetic field around a current-carrying wire?
a) Vernier caliper
b) Magnetic compass
c) Thermometer
d) Barometer
Ans: b) Magnetic compass
The compass needle deflects when near a current-carrying conductor.
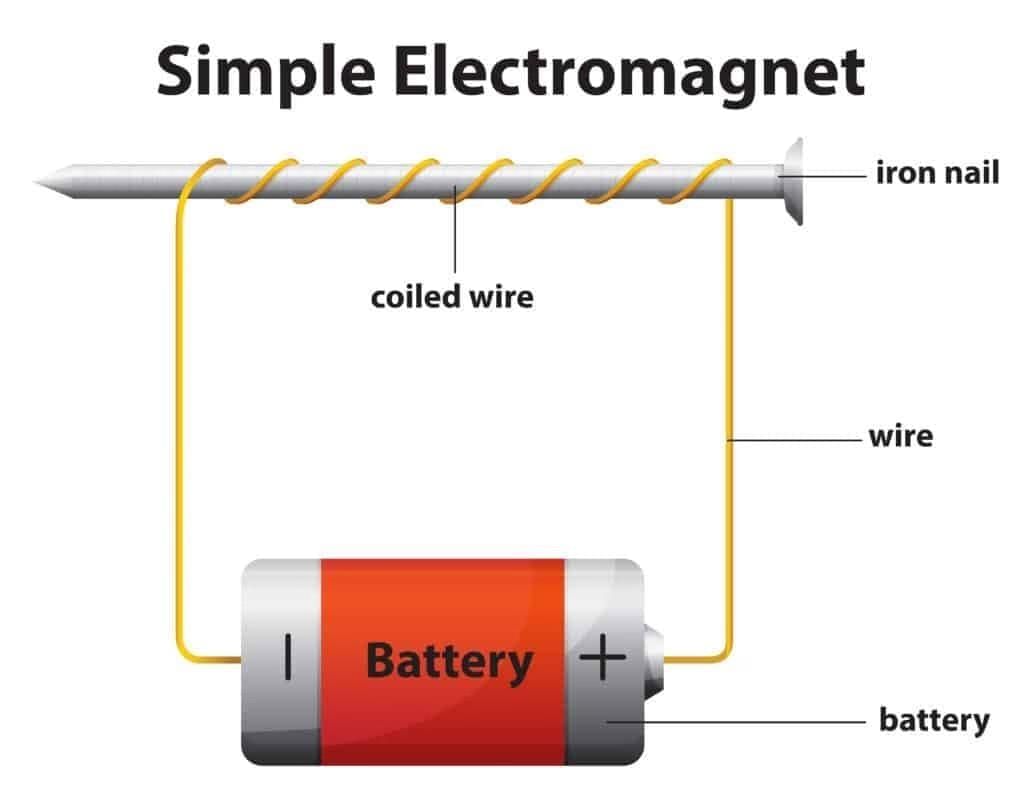
Q3. An electromagnet is:
a) A permanent magnet made of steel
b) A coil with an iron core that becomes a magnet when current flows
c) A bar magnet with fixed poles
d) A coil without any core
Ans: b) A coil with an iron core that becomes a magnet when current flows
Electromagnets work only when current is ON.
Q4. Which change will increase the strength of an electromagnet?
a) Using fewer turns of wire
b) Reducing current
c) Inserting a soft iron core
d) Removing the battery
Ans: c) Inserting a soft iron core
Strength increases with iron core, more turns, and higher current.
Q5. Lifting electromagnets are used mainly to:
a) Measure current
b) Lift and move heavy steel in scrap yards
c) Store electrical energy
d) Heat metals
Ans: b) Lift and move heavy steel in scrap yards
They can be switched ON to lift and OFF to release.
Fill in the Blanks
Instruction: Fill in the blanks with the correct word based on the chapter.
Q1. __________ wire is used as a heating element because it has high resistance.
Ans: Nichrome
High resistance produces more heat.
Q2. A device that produces electricity by a chemical reaction is called an electric __________.
Ans: cell
Cells convert chemical energy to electrical energy.
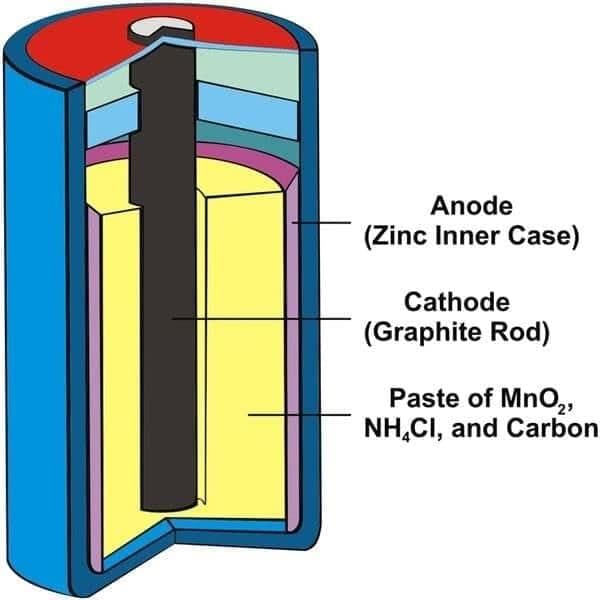
Q3. In a dry cell, the electrolyte is a moist __________.
Ans: paste
Hence called a “dry” cell.
Q4. In the lemon battery, the lemon juice acts as an __________.
Ans: electrolyte
The conducting liquid enables the reaction.
Q5. Multiple cells connected together form a __________.
Ans: battery
A battery supplies higher voltage/current than a single cell.
True or False
Q1. A nichrome wire becomes hot when current passes through it because of the heating effect of electric current.
Ans: True
Q2. Heat produced in a wire increases when the wire is longer, thinner, and carries more current.
Ans: True
Q3. A simple voltaic cell produces electricity through magnetic induction.
Ans: False (It is produced through chemical reactions between metals and the electrolyte.)
Q4. In a dry cell, the zinc container acts as the negative terminal.
Ans: True
Q5. Lithium-ion batteries are single-use and cannot be recharged.
Ans: False (They are rechargeable and widely used in phones and laptops.)
Very Short Answer Questions (1 line)
Instruction: Answer the following questions in one line.
Q1. What simple observation shows the magnetic effect of current?
Ans: A compass needle deflects near a current-carrying wire.
Q2. Name any one factor that increases the strength of an electromagnet.
Ans: Increasing the number of turns in the coil.
Q3. What happens to an electromagnet when the current is switched off?
Ans: It loses its magnetism.
Q4. What is resistance?
Ans: The opposition offered by a material to the flow of electric current.
Q5. Which terminal is at the center of a dry cell?
Ans: The carbon rod (positive terminal).
Short Answer Questions
Instruction: Answer the following questions in 2–3 lines.
Q1. State Oersted’s finding and its importance.
Ans: Oersted found that a current-carrying wire deflects a compass needle, proving that electric current creates a magnetic field. This linked electricity and magnetism and led to electromagnetism-based technologies.
Q2. How can you make a simple electromagnet at home?
Ans: Wrap insulated wire around a soft iron nail, connect the wire ends to a cell, and switch ON. The nail attracts iron objects while current flows; it stops when switched OFF.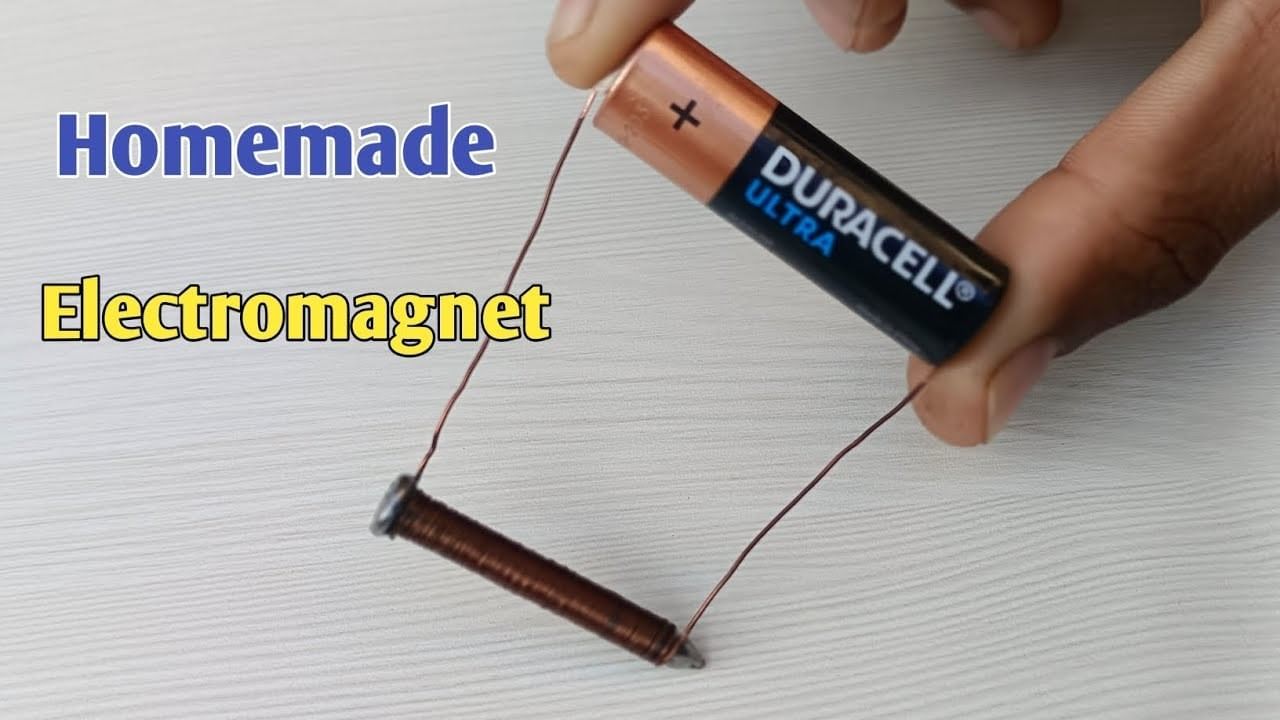
Q3. Mention two ways to increase the heat produced in a wire.
Ans: Use a longer/thinner high-resistance wire (like nichrome) and increase the current (more cells). Heat also increases with time of current flow.
Q4. What is the basic structure of a dry cell?
Ans: A zinc container (negative) filled with electrolyte paste and a central carbon rod (positive). It is a commonly used single-use cell.
Q5. How can you light an LED with lemons?
Ans: Insert a copper strip and an iron nail into each lemon (electrodes). Connect several lemon cells in series to increase voltage; ensure correct LED polarity.
Match the Following
Instruction: Match Column A with the correct option in Column B.
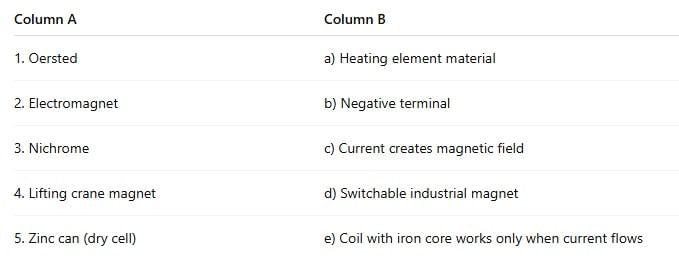
Ans:
Oersted — c) Current creates magnetic field
He discovered the magnetic effect of electric current.Electromagnet — e) Coil with iron core works only when current flows
It becomes magnetic only when powered.Nichrome — a) Heating element material
Nichrome’s high resistance makes it ideal for heaters.Lifting crane magnet — d) Switchable industrial magnet
Used to lift/drop heavy steel by switching current ON/OFF.Zinc can (dry cell) — b) Negative terminal
In a dry cell, the zinc container acts as the negative electrode.
FAQs on Worksheet Solutions: Electricity: Magnetic and Heating Effects - Worksheets with Solutions for Class 8
| 1. What are the magnetic effects of electric current? |  |
| 2. How does the heating effect of electric current work? |  |
| 3. What are some practical applications of the magnetic effects of electric current? |  |
| 4. What safety measures should be taken to avoid hazards related to electric current? |  |
| 5. Who discovered the relationship between electricity and magnetism? |  |