Writing Chemical Formula & Mole Concept | Science Class 9 PDF Download
Chemical Formula Of Common Compound
The chemical formula of a compound is a symbolic representation of its chemical composition. Chemical formulae provide insight into the elements that constitute the molecules of a compound and also the ratio in which the atoms of these elements combine to form such molecules.
For example, the chemical formula of water, which is H2O, suggests that two hydrogen atoms combine with one oxygen atom to form one molecule of water.
Importance of Chemical Formulae
- Chemical formulae provide insight into the chemical composition of a compound.
- They also represent the ratios in which the constituent elements combine to form the compound.
- The chemical formula of a compound is crucial while representing it in a chemical equation.
- Chemical formulae can also be employed to represent ions, free radicals and other chemical species.
Types of Chemical Formulae
While the term ‘chemical formula’ typically refers to the molecular formula of a compound (which denotes the total number of atoms of each constituent element in one molecule of the compound), the compositions of chemical compounds can be expressed in several ways, as listed in this subsection.
- Molecular Formula:
The molecular formula provides insight into the number of elements present in a compound. In molecular formulae, the elements are denoted by their respective symbols (as in the periodic table) and the number of atoms of each element in the molecule is written in subscript. For example- the molecular formula for glucose is C6H12O6. - Empirical Formula:
The empirical formula of a chemical compound represents the ratio of the elements present in that compound. Empirical formulae are usually obtained based on the analysis of experimental data. The empirical formula for glucose is CH2O. Empirical Formulae can be derived from the molecular formulae. - Structural Formula:
As the name suggests, the structural formula of a chemical compound provides insight into the arrangement of the atoms in the molecule.
How to write Chemical Formula
In order to write a chemical formula, it is important to know the symbol of the elements present in the compound, formula of the radicals and the valency of the elements in that compound. Following points should be kept in mind while writing a chemical formula.
- Most of the compounds are binary compounds, i.e. they have two elements.
- An atom with a positive charge is called a cation whereas an atom with a negative charge is called an anion
- For a compound containing a metal and a non-metal, the metal is named first, followed by the non-metal. For example: NaCl which consists of Na+ (metal ion) and Cl– (non-metal ion)
- Anions having -1 negative charge usually have a suffix as –ide. For example: F– – Floride
- Anions having oxyanions (oxygen + another element) usually have a suffix as –ate. For example – SO42- (Sulphate)
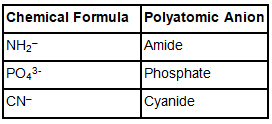
- When a polyatomic anion has H– ion, bi- or hydrogen is used as a suffix. For example – HCO3– - Bicarbonate or hydrogen carbonate
- Some polyatomic anions can be named as:
Solved Examples
Problem 1: In one molecule of the compound, determine how many atoms of every element are present for each one of these chemical formulas.
- HCN – hydrogen cyanide –It’s a toxic gas
- C18H21NO3 – codeine, a painkilling drug
- Ca10(PO4)6(OH)2 – Hydroxyapatite, that is present in the enamel of the tooth
Answer:
- One atom is present in each of the elements hydrogen, carbon and nitrogen, respectively. Remember that the subscript 1 is understood when no subscript is mentioned.
- This formula points out that in one molecule of the compound, 18 carbon atoms, 21 hydrogen atoms, one nitrogen atom and three oxygen atoms are existent.
- There are ten calcium atoms. The amounts of phosphorous, hydrogen and oxygen are affected by the subscripts outside the parentheses. There are six phosphorous atoms and two hydrogen atoms existent. Oxygen atoms exist in two positions in the formula. There are overall 26 oxygen atoms: two from the OH subunit (2×1) and 24 from the PO4 subunits (6×4)
Related Quantities and their Formulae
- Atomic and Molecular Mass
The atomic mass of an element is the mass of one atom of the element expressed in atomic mass units (amu). It accounts for the abundance of the various isotopes of the element and assigns an average value to the mass of one atom of the element.
For example, the atomic mass of carbon is 12.011 atomic mass units since carbon samples generally contain 98.89% of the carbon-12 isotope, 1.11% of carbon-13, and trace amounts of carbon-14. However, the atomic masses of these isotopes are different.
The atomic mass of a carbon-12 atom is 12 atomic mass units, but that of a carbon-13 atom is 13 amu. The atomic mass of an element is roughly equal to the sum of all the protons and neutrons present in its nucleus.
The molecular mass of an element is the sum of the atomic masses of all its constituent elements. This quantity is also represented in terms of atomic mass units. Therefore, the molecular mass of water is equal to the sum of the atomic masses of its constituents – hydrogen and oxygen.
The atomic mass of hydrogen is 1.00794 amu and that of oxygen is 15.9994. Since water molecules contain 2 hydrogen atoms and only one oxygen atom, the molecular mass of H2O is 18.0154 amu. - Molar Mass
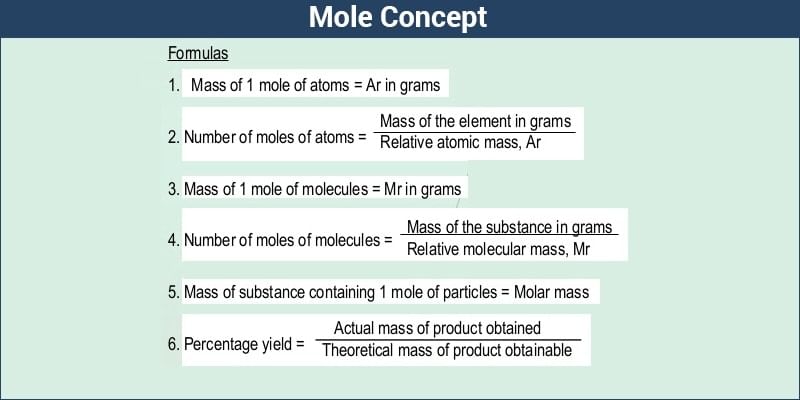
The molar mass of a substance is defined as the total mass of one mole of the substance. It is often represented in terms of ‘grams per mole’ (g/mol). However, the SI unit of this quantity is kg/mol. Molar mass can be represented by the following formula:
Molar mass of a Substance = (Mass of the Substance in grams)/(Number of Moles)
For example, the molar mass of water is approximately 18.015 g/mol, which is the mass of NA number of water molecules. - Gram Atomic Mass and Gram Molecular Mass
The gram atomic mass of an element is the mass of one mole of that element. Similarly, the gram molecular mass of a compound refers to the mass of a single mole of the compound. Therefore, the gram atomic mass of hydrogen is approximately 1.007g and the gram molecular mass of water is approximately 18.015g. - Related Formulae
The number of moles in a given sample of an element/compound can be calculated by dividing the total mass of the sample by the molar mass of the element/compound, as described by the following formula.
Number of Moles = (Mass of the Sample)/(Molar Mass)
The total number of atoms/molecules in a sample can be calculated by multiplying the number of moles with the Avogadro constant. This formula can be written as:
Number of Atoms or Molecules = (Number of Moles)*(6.022*1023)
The relationship between the atomic mass unit (amu) and the gram is given by:
1 amu = (1gram)/(6.022*1023) = 1.66*10-24 grams
Therefore, the mass of one mole of an element will be equal to its atomic mass in grams.
 Formulas of Mole Concept
Formulas of Mole Concept
Solved Examples on the Mole Concept
Some solved example questions on the mole concept are provided in this subsection.
Q.1: How many moles of iron are present in a pure sample weighing 558.45 grams?
Ans: The molar mass of iron is 55.845 g/mol. Therefore, the number of moles of iron in the pure sample weighing 558.45 grams is: = 10 moles.
Q.2: How many molecules of water are present in 36 grams of water?
Ans: The molar mass of water is 18 (approximately). Therefore, 36 grams of water makes up a total of 2 moles. Each mole has 6.022*1023 water molecules. The total number of H2O molecules in 36 grams of water is: 12.044*1023
Q.3: How many grams of carbon can be found in 1 mole of carbon dioxide?
Ans: 1 mole of CO2 contains 1 mole of carbon and 2 moles of oxygen. The molar mass of carbon is 12.0107 g/mol. Therefore, 1 mole of CO2 contains 12.01 grams of carbon and 32 grams of oxygen.
Frequently Asked Questions – FAQs
Q. What is a mole equal to?
Ans: One mole of a substance is equal to the substance’s 6.022 x 1023 units (such as atoms, molecules, or ions). The 6.022 x 1023 number is known as the number of Avogadro or the constant of Avogadro. For the conversion of mass and number of particles, the definition of the mole can be used.
Q. What is the importance of the mole concept?
Ans: All chemistry is pervaded by the mole definition. Since most quantitative chemical calculations are focused on the mole, for the study of chemistry, an understanding of the mole is important. A knowledge of how the mole applies to mass, number of entities
Q. What is a mole in chemistry?
Ans: In chemistry, the mole, also written mol., is a standard scientific unit for the calculation of large amounts of very small entities, such as atoms, molecules, or other objects.
Q. Why do we use mole fraction?
Ans: The mole fraction defines the number of single component molecules (or moles) divided by the total number of molecules (or moles) in the mixture. When two reactive components are mixed together, the mole fraction is useful, as the ratio of the two components is understood if the mole fraction of each is known.
Q. Is mole fraction equal to partial pressure?
Ans: In a mixture, the partial pressure of each gas is proportional to the mole fraction. The pressure exerted by each gas (its partial pressure) in the gas mixture is independent of the pressure exerted by all other gases present in the gas mixture.
Mole Concept (Old Syllabus)
What is the Mole Concept?
The mole concept is a convenient method of expressing the amount of a substance. Any measurement can be broken down into two parts – the numerical magnitude and the units that the magnitude is expressed in. For example, when the mass of a ball is measured to be 2 kilograms, the magnitude is ‘2’ and the unit is ‘kilogram’.
When dealing with particles at an atomic (or molecular) level, even one gram of a pure element is known to contain a huge number of atoms. This is where the mole concept is widely used. It primarily focuses on the unit known as a ‘mole’, which is a count of a very large number of particles.
What is a Mole?
In the field of chemistry, a mole is defined as the amount of a substance that contains exactly 6.02214076 * 1023 ‘elementary entities’ of the given substance.
The number 6.02214076*1023 is popularly known as the Avogadro constant and is often denoted by the symbol ‘NA’. The elementary entities that can be represented in moles can be atoms, molecules, monoatomic/polyatomic ions, and other particles (such as electrons).
For example, one mole of a pure carbon-12 (12C) sample will have a mass of exactly 12 grams and will contain 6.02214076*1023 (NA) number of 12C atoms. The number of moles of a substance in a given pure sample can be represented by the following formula:
n = N/NA
Where n is the number of moles of the substance (or elementary entity), N is the total number of elementary entities in the sample, and NA is the Avogadro constant.
The word “mole” was introduced around the year 1896 by the German chemist Wilhelm Ostwald, who derived the term from the Latin word moles meaning a ‘heap’ or ‘pile.
The number of moles of a molecule may not always be equal to the number of moles of its constituent elements. For example, a mole of water contains NA number of H2O molecules. However, each water molecule contains 2 hydrogen atoms and one oxygen atom. Therefore, one mole of H2O contains 2 moles of hydrogen and one mole of oxygen.
|
84 videos|478 docs|60 tests
|
FAQs on Writing Chemical Formula & Mole Concept - Science Class 9
| 1. What is the chemical formula for water? |  |
| 2. What is the chemical formula for table salt? |  |
| 3. What is the chemical formula for carbon dioxide? |  |
| 4. How is the chemical formula of a compound determined? |  |
| 5. What is the significance of the chemical formula in understanding a compound? |  |






















