|
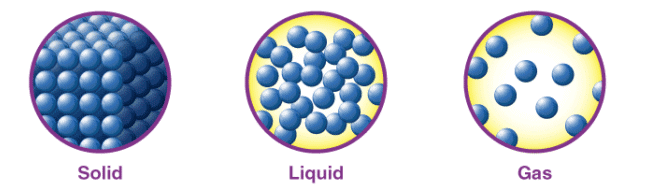
In the solid state, particles are closely packed in a fixed, orderly arrangement, have a definite shape and volume, and exhibit limited movement due to strong intermolecular forces. |
Card: 4 / 28 |
|
False. Gases have neither a definite shape nor volume; they expand to fill their container. |
Card: 6 / 28 |
|
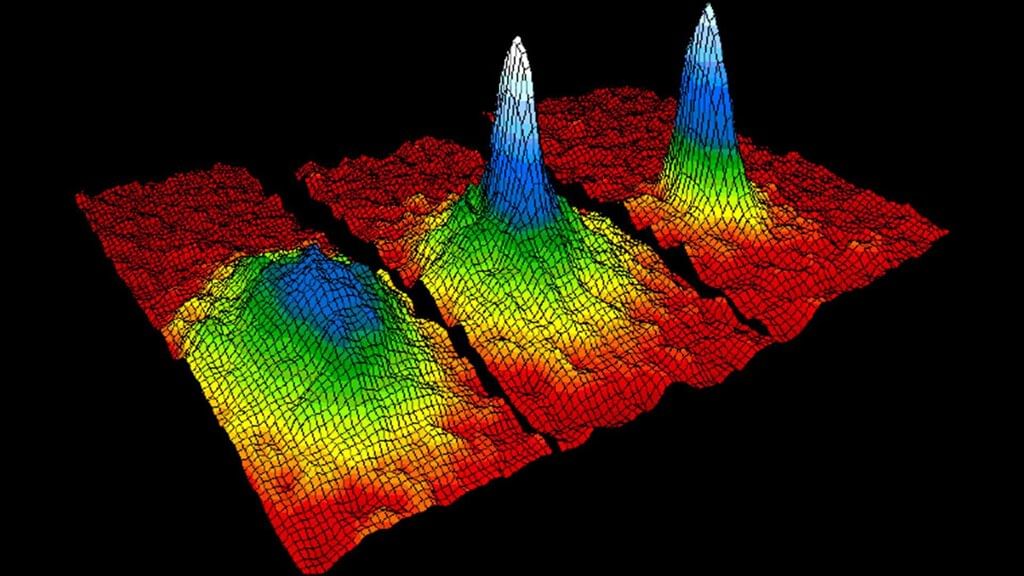
What occurs in Bose-Einstein Condensate (BEC) at temperatures close to absolute zero? |
Card: 7 / 28 |
|
Particles occupy the same space and quantum state, acting as a single quantum entity. |
Card: 8 / 28 |
|
Fill in the blank: The temperature at which a solid turns into a liquid is known as the ___. |
Card: 9 / 28 |
|
The modern atomic theory states that atoms are the smallest units of matter that ___ and ___ when chemical reactions occur. |
Card: 13 / 28 |
|
False; J.J. Thomson proposed the 'plum pudding' model, while Ernest Rutherford proposed the nuclear model. |
Card: 16 / 28 |
|
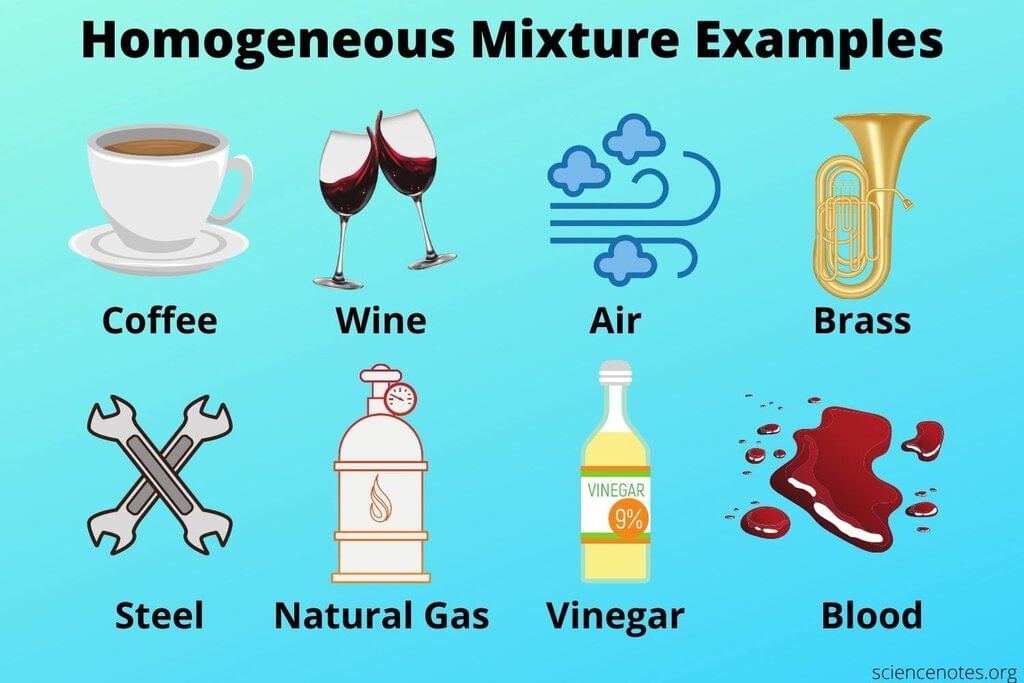
A homogeneous mixture has a uniform composition throughout, while a heterogeneous mixture retains distinct individual properties. |
Card: 18 / 28 |
|
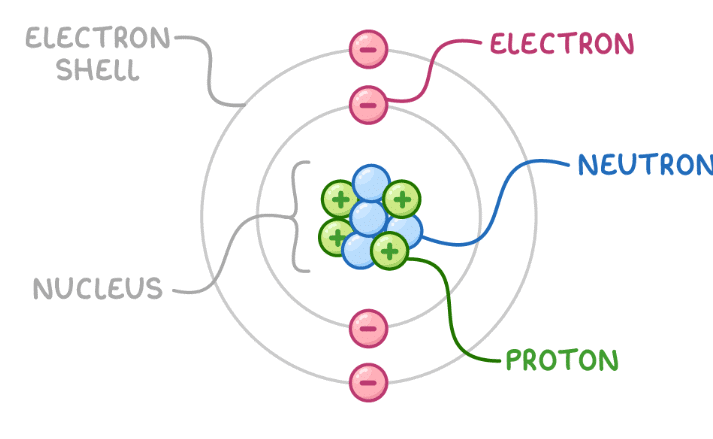
Atoms consist of a nucleus containing ___ and ___, surrounded by ___ in various energy levels. |
Card: 19 / 28 |
|
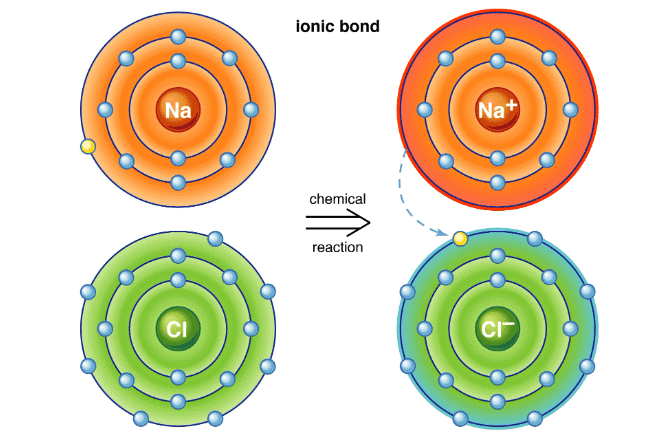
True or False: Ionic bonds are formed by the sharing of electrons between atoms. |
Card: 21 / 28 |
|
False; Ionic bonds are formed by the transfer of electrons from one atom to another. |
Card: 22 / 28 |
|
True or False: The atomic radius increases across a period from left to right. |
Card: 23 / 28 |
|
False - The atomic radius decreases across a period due to increasing nuclear charge. |
Card: 24 / 28 |
|
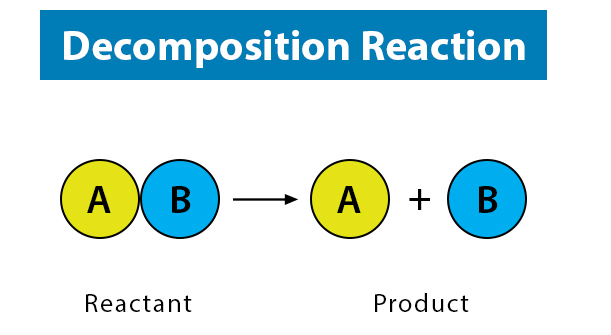
True or False: In a decomposition reaction, a single compound breaks down into two or more products. |
Card: 27 / 28 |
 Completed! Keep practicing to master all of them. |