Class 9 Science - Is Matter Around Us Pure Previous Year Questions
Short Answer Type Questions
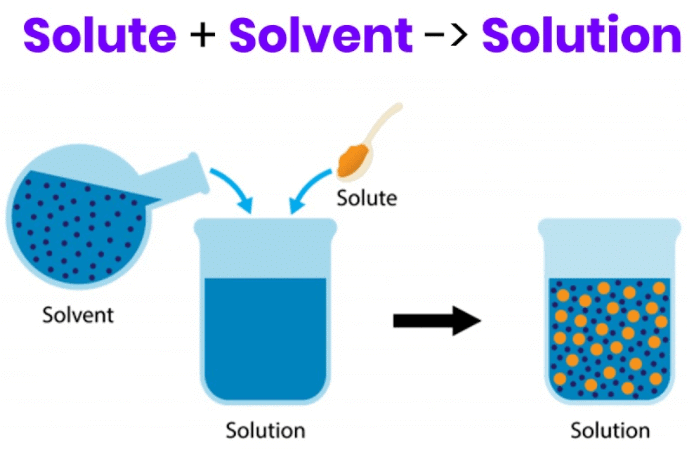
Q.1. What are the components of a solution? [2024]
Ans.
- Solute
- Solvent
Q.2. Name a process by which pure solid can be separated from solution in crystal form. [2024]
Ans. Crystallization
Q.3. Name the process by which cream is separated from milk. [2024]
Ans. Centrifugation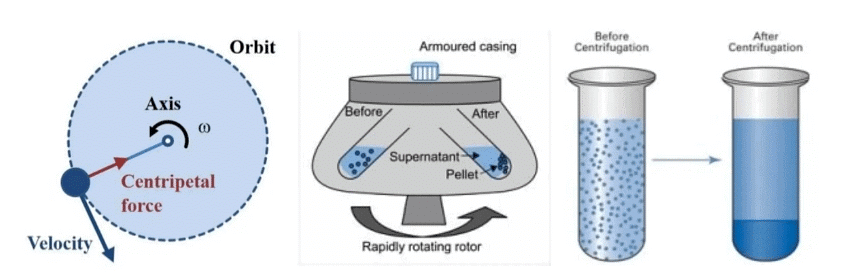 Q.4. Air is a chemical compound or a mixture? Support your answer with suitable facts. [2024]
Q.4. Air is a chemical compound or a mixture? Support your answer with suitable facts. [2024]
Ans. Air is a mixture having nitrogen and oxygen as the main components.
The following facts support that air is a mixture:
- The air contains various gases the proportion of which varies from place to place.
Example: The air around the city has less oxygen than the air around a village or an open land. - When the gases such as oxygen, nitrogen, and carbon dioxide are mixed to obtain a sample of air, no heat, light, or electricity is evolved or absorbed.
- The various gases found in the air can easily be separated by fractional distillation of liquid air.
- Air retains the properties of its constituents.
Q.5. Why is crystallization better than evaporation for the separation of mixtures? [2023]
Ans.
- In crystallization, we obtain the components in the form of crystals which are the purest form of a substance, and impurities are left in the mother liquor. Crystals are separated from the mother liquor.
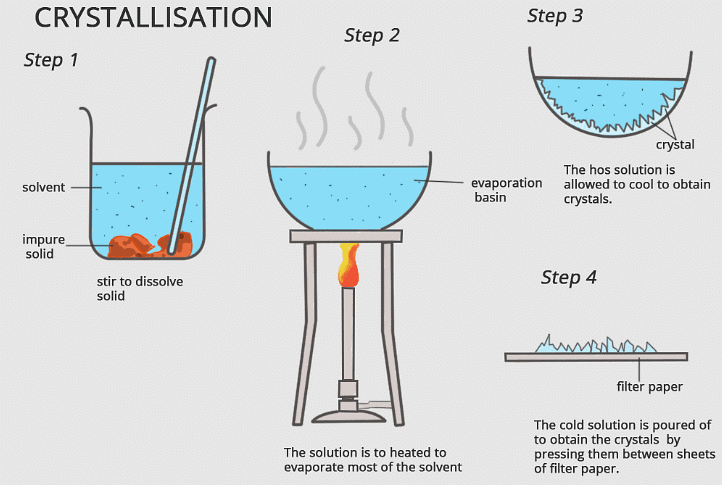
- This is not possible in evaporation. Some solids may decompose on heating to dryness. Such solids cannot be purified by evaporation.
Q.6. List two points of difference between homogeneous and heterogeneous mixtures. [2023]
Ans.
- Homogeneous mixtures have uniform composition throughout whereas heterogeneous mixtures have non-uniform composition.
- Homogeneous mixtures consist of only one phase whereas heterogeneous mixtures have more than one phase.
Q.7. List four properties of nonmetals. Give two examples. [2023]
Ans. Properties of non-metal:
(i) They show a variety of colors.
(ii) They are poor conductors of heat and electricity.
(iii) They are not malleable or ductile.
(iv) They are not lustrous or sonorous.
Example: Sulphur and phosphorus.
Q.8. Name the separation technique by which we can obtain colored components from ink. Give two more applications of the technique used. [2022]
Ans. The technique used to separate colored components of ink is called chromatography.
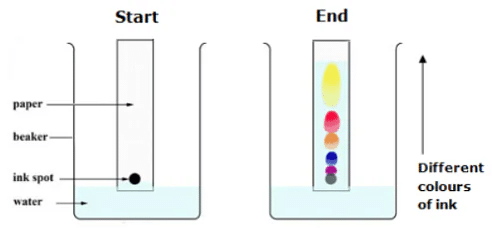
Applications of chromatography:
(i) To separate a mixture of components soluble in the same solvent.
(ii) To separate mixtures of vitamins and amino acids.
Q.9. Explain the term centrifugation? Give one of its applications. [2022]
Ans. Churning at high speed, denser particles settle at the bottom, separating cream from milk.
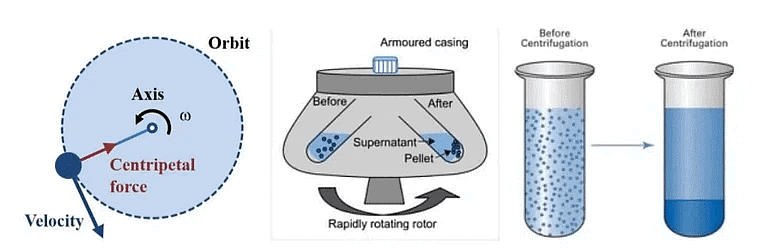
Application: Washing machines and urine test.
Q.10. Write down the processes involved in sequential order to get the supply of drinking water to your home from the waterworks. [2021]
Ans. Reservoir → Sedimentation tank → Loading tank → Filtration tank → Chlorination kills bacteria → To Home.
- Water is passed through a sedimentation tank in which heavy impurities settle down due to gravity.
- Loading tank contains potash alum which helps in making sedimentation faster by suspending impurities that are lighter.
- The filtration tank removes insoluble suspended impurities.
- Chlorination tank is used to disinfect water and make it fit for drinking which is supplied to our homes.
Q.11. Two students A and B were given 10 ml of water in a bowl and a plate respectively. They were told to observe the rate of evaporation. Name the student whose water evaporates faster and explain its reason. [2020]
Ans.
- The water of 'B' will evaporate faster. It is because the surface area is more in the plate. Therefore, the rate of evaporation becomes faster.
- The rate of evaporation depends upon surface area. The greater the surface area more will be the rate of evaporation.
- That is why we drink hot tea from a saucer easily than from a cup.
Q.12. Why is the inter-conversion of states of matter considered a physical change? Give three reasons to justify your answer. [2020]
Ans. The interconversion of states of matter is considered a physical change because:
(i) It occurs without a change in composition.
(ii) The substances differ in physical properties but chemically they are the same.
Example: Water changes into ice below 0°C. Ice changes into liquid above 0°C. Liquid water changes into steam at 100°C.
Physical states of water are different due to different forces of attraction and intermolecular spaces, but the composition is the same, i.e. all of them contain same water molecules.
(iii) No new substance with new properties will form.
Q.13. (a) Define an element.
(b) What is meant by malleability? Name any two substances that are malleable. [2018]
Ans. (a) Element is a substance that is made up of only one kind of atom.
(b) Malleability is a property due to which a metal can be beaten into sheets.
 Example: Gold and Silver.
Example: Gold and Silver.
Q.14. Describe an activity to show that particles of matter have spaces between them. [2018]
Ans. The activity is performed as under:
(i) Take a 100 mL beaker.
(ii) Fill half of the beaker with water and mark the level of water with a pen.
(iii) Dissolve about 5 g of salt into the water with the help of a glass rod.
(iv) Observe the change in the level of water.
- We find that there is no change in the level of water in the beaker. That means the particles of salt have occupied the empty spaces between particles of water, so no change in water level has taken place.
- The activity proves that particles of matter have empty spaces between them.
Q.15. (a) Write any two differences between a chemical change and a physical change.
(b) State one instance in which water undergoes a physical change and one in which it undergoes a chemical change. [2018]
Ans.
(a) Two differences between chemical change and physical change:
(i) No new substance is formed in a physical change.
Example: When the ice melts into water, it is a physical change because ice and water are the same substances chemically.
But in a chemical change, a new substance is formed which has properties different from reactants.
Example: When Iron and Sulphur are heated, iron sulphide is obtained having properties different from Iron and Sulphur.
(ii) Product in a physical change can be converted back to the original substance.
Example: When the ice melts to water, the latter can be converted back to ice on cooling.
But the product in a chemical change cannot be reconverted in the original substances.
Example: Iron sulphide cannot be converted back into iron and Sulphur easily.
(b)
- Water on heating to 100°C is converted into steam. It is a physical change.
- On passing an electric current through water, we obtain hydrogen and oxygen gases. This is a chemical change.
Q.16. What is meant by an aqueous and non-aqueous solution? Give one example each. [2017]
Ans. A solution that is prepared in water is called an aqueous solution.
Example: A solution of sodium chloride in water.
A solution that is prepared in a solvent other than water is called a non-aqueous solution.
Example: A solution of iodine in carbon tetrachloride.
Q.17. How is the heating of sugar and heating of ammonium chloride different from each other? Explain your answer. [2017]
Ans. Sugar on heating decomposes to give simpler products while ammonium chloride on heating sublimes to give vapours of ammonium chloride without giving molten ammonium chloride. The sublimed vapours can be collected in an inverted funnel to give back solid ammonium chloride.
Q.18. What are heterogeneous mixtures? [2017]
Ans. Those mixtures whose composition is not uniform throughout are called heterogeneous.
Q.19. A solution of alcohol in water has been prepared by mixing 150 ml of alcohol with 600 ml of water. Calculate the volume. Percentage of the Solution. [2017]
Ans. % by volume
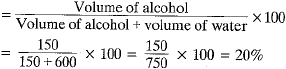
Q.20. Differentiate between an element and a compound (any two-point). Write one example of each. [2016]
Ans.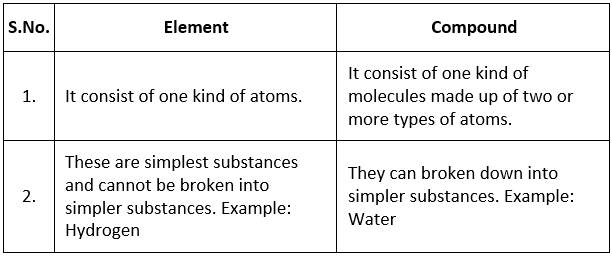 Q.21. (a) Name the separation technique you would follow to separate:
Q.21. (a) Name the separation technique you would follow to separate:
(i) Dyes from black ink
(ii) A mixture of salt and ammonium chloride
(iii) Cream from milk
(iv) Sodium chloride from its solution in water
(b) State the principle used in separating a mixture of two immiscible liquids. [2016]
Ans. (a) (i) Chromatography
(ii) Sublimation
(iii) Centrifugation
(iv) Evaporation
(b) The principle used in the separation of immiscible liquids by separating funnel, based on the difference in their densities. The heavier liquid will form the lower layer, which will get separated first. The lighter liquid will form the upper layer, so it will get separated later.
Q.22 Why copper sulphate solution in water does not show Tyndall effect but a mixture of water and milk shows. [2016]
Ans.
- Copper sulphate solution does not show Tyndall effect because particles are very small and do not cause scattering of light.
- Water and milk form a colloidal solution which shows Tyndall effect because particles are larger, which causes scattering of light and shows the Tyndall effect.
Q.23. Name the separation technique by which we can obtain coloured components from ink? Give two more applications of the technique used. [2016]
Ans. Chromatography is used to obtain coloured components from ink.
Applications:
- Pigments from natural color can be separated by chromatography.
- Drugs from blood can be separated by chromatography.
Q.24. How many liters of 15% (mass/volume) sugar solution would it take to get 75 g of sugar. [2016]
Ans. Mass by volume %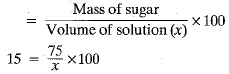
► x = 500 mL ; x = 0.5 L
Long Answer Type Questions
Q.1. Rahul’s mother mixed oil and water in the kitchen by mistake. Rahul told her that he can separate the mixture. Name the technique used by Rahul and explain how he will do it. Draw the diagram and write the principle of this technique. [2021]
Ans.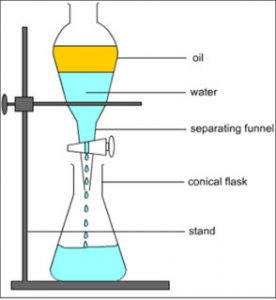
(i) The technique is called gravity separation by using a separating funnel.
(ii) He will put the mixture of liquids in a separating funnel.
(iii) Oil and water will form a separate layer. The lighters layer forms an upper layer, heavier (water) will form the lower layer.
(iv) When the stop cock of the separating funnel is opened, water will come out.
(v) Close the stop cock.
(vi) When the stop cock is opened again, the oil will come out and both will get separated.
(vii) This process is based on the principle of difference in the density of two liquids.
Q.2. Write your observation when the following processes take place.
(a) An aqueous solution of sugar is heated till it gets dried up.
(b) A saturated solution of KCl at 60°C is allowed to cool at room temperature.
(c) A mixture of iron filings and Sulphur powder is heated strongly.
(d) A beam of light is passed through a colloidal solution.
(e) Dil. HCI is added to a mixture of iron filings and sulphur powder. [2021]
Ans. (a) Sugar remains as a residue in the form of solid mass.
(b) Crystal of KCI are formed.
(c) A black coloured solid called iron sulphide is formed.
(d) The path of light becomes clearly visible due to the scattering of light by colloidal particles.
(e) A colorless and odorless hydrogen gas is evolved.
Q.3. The boiling point of alcohol is 78°C and that of water is 100°C. Explain the separation technique will you use to separate them from a mixture? Which liquid will be separated first and which will be left behind? Draw a diagram to show the apparatus and the setup used in the process. [2019]
Ans. The process used is distillation. It is a double process of evaporation followed by distillation.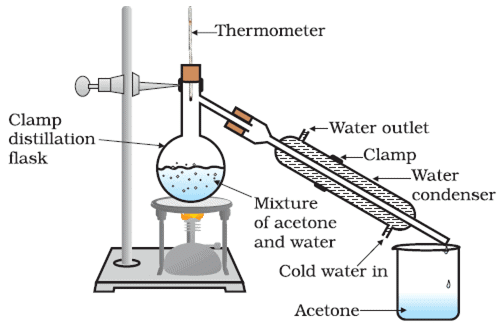 (i) Take a mixture of alcohol and water in the distillation flask.
(i) Take a mixture of alcohol and water in the distillation flask.
(ii) Set the apparatus as shown in the diagram.
(iii) Start the flow of water into the condenser.
(iv) Start heating with the help of the burner.
(v) Note down the continuous temperature.
(vi) At 78 °C, alcohol will change into vapour completely and get the condenser to get pure alcohol.
Alcohol will be separated first, whereas water will be left behind.
Q.4. (a) You are given a mixture of sand, water, and mustard oil. How will you separate the components of this mixture? Explain it with the help of different separation methods involved in it.
(b) Give a flow diagram showing the process of obtaining gases from the air. [2018]
Ans. (a)
- Filter the mixture. Sand will be residue. Mustard oil and water will be filtrate
- Take mustard oil and water in a separating funnel.
- Open the stop cock, water will come out first. Collect it in a beaker.
- Mustard oil will be left in the separating funnel and get separated.
(b)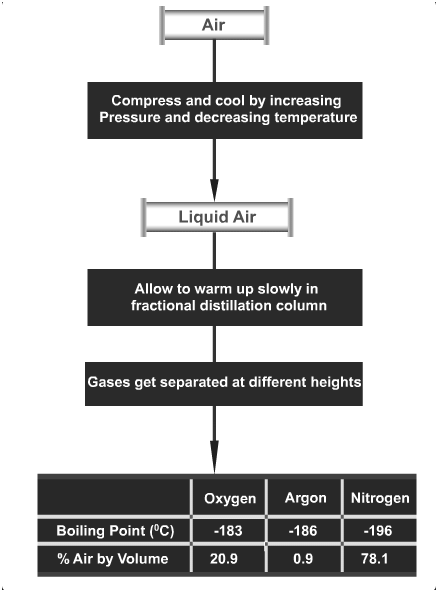
Q.5. Show diagrammatically how water is purified in the waterworks system and list the processes involved. [2016]
Ans.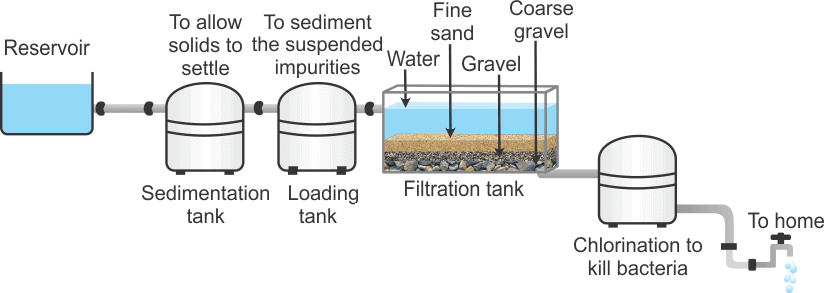 Process: (i) Sedimentation (ii) Loading (iii) Filtration (iv) Chlorination Q.6. Describe any three properties of colloids. Categories the following examples of colloids into different categories of colloids:
Process: (i) Sedimentation (ii) Loading (iii) Filtration (iv) Chlorination Q.6. Describe any three properties of colloids. Categories the following examples of colloids into different categories of colloids:
jelly, fog, milk, shaving cream. [2016]
Ans. Properties of colloids:
- The size of particles of a colloid is too small to be seen individually by naked eyes.
- Colloids are big enough to scatter a beam of light passing through it and make its path visible.
- They do not settle down when left undisturbed, that is, a colloid is quite stable.
Categories:
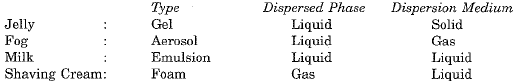
|
85 videos|548 docs|60 tests
|
FAQs on Class 9 Science - Is Matter Around Us Pure Previous Year Questions
| 1. What is meant by the term "pure substance"? |  |
| 2. How can we differentiate between a mixture and a pure substance? |  |
| 3. What are the different types of mixtures? |  |
| 4. What methods can be used to separate components of a mixture? |  |
| 5. Why is it important to study the purity of substances in chemistry? |  |

















