Class 10 Science Previous Year Questions - Periodic Classification of Elements
Q1. Which physical and chemical properties of the elements were used by Mendeleev in creating his periodic table ? List two observation which posed a challenge to Mendeleev's periodic law. (C.B.S.E. 2008)
Ans. The creation of Mendeleev's periodic table was based upon certain physical and chemical properties.
Physical properties : The atomic masses of the elements was taken into account and the elements were arranged in order of increasing atomic masses. The influences of their physical properties such as melting points, boiling points, density etc. were considered.
Chemical properties : The distribution of the elements into different groups was linked with formation of hydrides by combining with hydrogen and formation of oxides by combining with oxygen. This is linked with the valency of the elements.
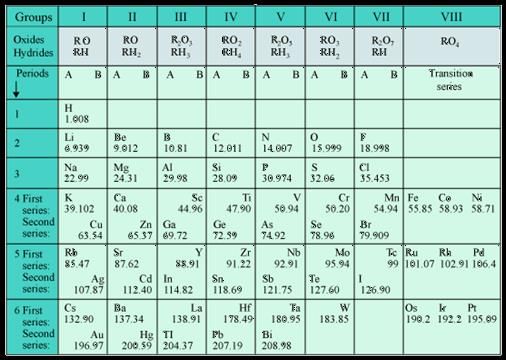 The two main observations which posed challenge to Mendeleev’s periodic table are as follows:
The two main observations which posed challenge to Mendeleev’s periodic table are as follows:
(i) Position of isotopes : Since the isotopes of an element differ in their atomic masses, they must be assigned separate slots or positions in the periodic table.
(ii) Anomalous positions of some elements : In the Mendeleev’s periodic table, certain elements with higher atomic masses precede or placed before the elements with lower atomic masses. For example,the element Ar (Atomic mass = 39.9) is placed before the element K (Atomic mass = 39.1)
Q2. Using the part of the periodic table given below, answer the questions that follow.
| Group Period | I | II | III | IV | V | VI | VII | Zero |
| 1 | H | He | ||||||
| 2 | Li | Be | B | C | N | O | F | Ne |
| 3 | Na | Mg | Al | Si | P | S | Cl | Ar |
| 4 | K | Ca |
(i) Na has physical properties similar to which elements and why?
(ii) Write the electronic configuration of N and P
(iii) State one property common to fluorine and chlorine. (C.B.S.E. All India 2008)
Ans. (i) Na has physical properties similar to Li and K. All the three elements have one electron each in the valence of their atoms. These are known as alkali metals.
(ii) Electronic configuration of N (z = 7) = 2, 5
Electronic configuration of P (z = 15) = 2, 8, 5
(iii) Both the elements have seven electrons in the valence shells as their atoms.
Fluorine (z = 19) = 2, 7
Chlorine (z = 17) = 2, 8, 7
Q3. Table given below shows a part of the periodic Table
| H | He | ||||||
| Li | Be | B | C | N | O | F | Ne |
| Na | Mg | Al | Si | P | S | Cl | Ar |
Using this table explain why
(a) Li and Na are considered as active metals.
(b) Atomic size of Mg is less than that of Na.
(c) Fluorine is more reactive than chlorine.
Ans. (a) Both Li and Na are active elements since their atoms have only one, electron in their valence shells. They readily lose this electron to have the configuration of the nearest noble gas element.
(b) Mg is placed after Na in the same period (third). As the atomic size decreases along a period, the size of Mg is less than that of Na.
(c) Both F and CI belong to group 17 (halogen family). Since fluorine is more electronegative than chlorine, it is therefore more reactive also.
Q4. (a) Why do all the elements of the same group have similar properties ?
(b) How will the tendency to gain electrons change as we go from left to right across a period ? Why ?
Ans. (a) The properties of the elements are linked with the valence shell electronic configuration of their atoms. The elements with the same configuration are expected to have similar properties. In a group, the elements are separated by definite gaps of atomic numbers and have same number of electrons in the valence shells of their atoms. For example, the alkali metals in group I have one electron each. They have similar properties. For further details, consult text part.
(b) In moving from left towards the right across a period, the tendency of the elements to gain electrons increases.
Explanation. In general, the atoms of all the elements have a desire or urge to have stable electronic configuration of the nearest noble gas elements or to have eight electrons in their outermost or valence shells. Now, across a period the valence electrons are added one by one from left to the right. This is supported by the electronic configuration of the elements present in period 3 or third period.
| Element | Na | Mg | Al | Si | P | S | Cl | Ar |
| No. of valence electrons | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| No. of electrons needed in valence shells | 7 | 6 | 5 | 4 | 3 | 2 | 1 | 0 |
This clearly shows that the element chlorine needs one electron while oxygen requires two to have to stable electronic configuration. Thus, tendency to gain electrons increases from left to right across a period.
Some Important Points to Remember
• Elements are classified on the basis of similarities in their physical and chemical properties.
• Dobereiner grouped elements into triads.
• Newland grouped elements on the basis of law of octaves.
• Mendeleev grouped elements in the increasing order of their atomic masses and the similarity in chemical properties.
• Mendeleev was able to predict the existence of some elements on the basis of gaps in the periodic table.
• Moseley discovered that fundamental property of an element is its atomic number, rather than atomic mass. He revised Mendeleev Periodic Table on the basis of atomic numbers of elements and removed some of its anomalies.
• Elements in the long form of Modern Periodic Table are arranged in 18 vertical columns called groups and 7 horizontal rows called periods.
• The elements arranged in the long form of periodic table show (i) periodicity of properties (ii) atomic number.
|
80 videos|359 docs|87 tests
|
FAQs on Class 10 Science Previous Year Questions - Periodic Classification of Elements
| 1. What is the periodic classification of elements in Class 10? |  |
| 2. What are the benefits of studying periodic classification of elements in Class 10? |  |
| 3. What are some common properties used in the periodic classification of elements in Class 10? |  |
| 4. How do you determine the position of an element in the periodic table in Class 10? |  |
| 5. What are some examples of elements that belong to the same group in the periodic table in Class 10? |  |

















