Solved Examples - Solutions (Part - 3) | Additional Study Material for JEE PDF Download
Example 27. The density of a 3M sodium thiosulphate solution (Na2S2O3) is 1.25 g/mL. Calculate
(i) the percentage by mass of sodium thiosulphate,
(ii) the mole fraction of sodium thiosulphate and
(iii) molalities of Na and S2O32-ions -
Solution.
(i) Mass of 1000 mL of Na2S2O3 solution
= 1.25 × 1000 = 1250 g
Mass of Na2S2O3 in 1000 mL of 3M solution
= 3 × Mol. mass of Na2S2O3
= 3 × 158 = 474 g
Mass percentage of Na2S2O3 in solution
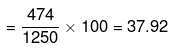
Alternatively,
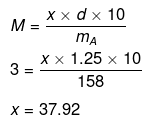
(ii) No. of moles of Na2S2O3 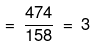
Mass of water = (1250 - 474) = 776 g
No. of moles of water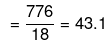
Mole fraction of Na2S2O3
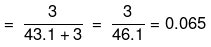
(iii) No. of moles of Na+ ions
= 2 × No. of moles of Na2S2O3
= 2 × 3 = 6
Molality of Na ions
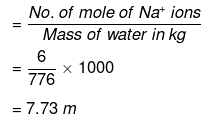
No. of moles of S2 ions = No. of moles of Na2S2O3 = 3
ions = No. of moles of Na2S2O3 = 3
Molality of  ions
ions
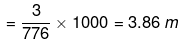
Example 28. A solution is perpared by dissolving 5.64 gm of glucose in 60g of water. Calculate the following.
(i) mass per cent of each of glucose and water,
(ii) molality of the solution,
(iii) mole fraction of each of glucose and water.
Solution.
(i) Total mass of solution
= 5.64 60 = 65.64 g
Mass per cent of glucose
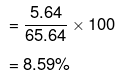
Mass per cent of water
= (100 -Mass per cent of glucose)
= (100 -8.59) = 91.41%
(ii) No. of moles of glucose
= 
Mass of water in kg
= 
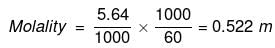
(iii) No. of moles of glucose
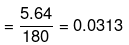
No. of moles of water
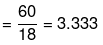
Mole fraction of glucose
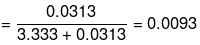
Mole fraction of water
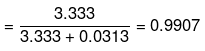
Example 29. The volume of water which must be added to a mixture of 350 cm3 of 6 M HCl and 650 ml of 3 M HCl to get a resulting solution of 3 M concentration is [Ans. D]
(a) 75 mL
(b) 150 mL
(c) 100 mL
(d) 350 mL
Ans. (d)
Solution.
Molarity of mixture of 6 M and 3 M HCl
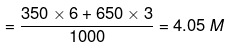
Now, apply dilution formula
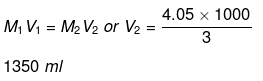
Volume of water to be added
= 1350 - 1000 = 350 ml
Example 30. The mole fraction of CH3OH in an aqueous solution is 0.02 and its density is 0.994 g cm-3. Determine its molarity and molality.
Solution. Let x mole of CH3OH and y mole of water be present in solution.
Mole fraction of CH3OH
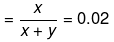
So,

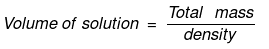
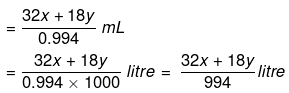
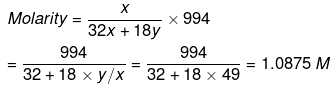
Example 31. Calculate the concentration of NaOH solution in g/mL which has the same normality as that of a solution of HCl of concentration 0.04 g/mL. -
Solution.
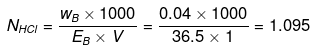
NNaOH º NHCl
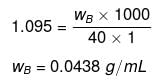
Example 32. How many Na+ ions are present in 50 mL of a 0.5 M solution of NaCl ?
Solution.
Number of moles of NaCl
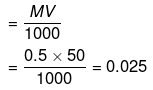
NaCl → Na+ + Cl¯
Number of moles Na = Number of moles of NaCl = 0.025
Number of ions of Na = 0.025 × 6.023 × 1023 = 1.505 × 1022
Example 33. 250 mL of a Na2CO3 solution contains 2.65 g of Na2CO3. 10 mL of this solution is added to x mL of water to obtain 0.001 M Na2CO3 solution. The value of x is :
(Molecular mass of Na2CO3 = 106 amu)-
(a) 1000
(b) 990
(c) 9990
(d) 90
Ans. (b)
Solution.
Molarity of solution

M1V1 = M2V2
0.1 × 10 = 0.001 (10 x)
x = 990 mL
Example 34. The volumes of two HCl solutions A (0.5 N) and B (0.1 N) to be mixed for preparing 2 L of 0.2 N HCl are -
(a) 0.5 L of A 1.5 L of B
(b) 1.5 L of A 0.5 L of B
(c) 1 L of A 1 L of B
(d) 0.75 L of A 1.25 L of B
Ans. (a)
Solution.
Let x L of A and (2 -x) L of B are mixed.
M1V1 M2V2 = MR (V1 V2)
0.5 × x 0.1 (2 -x) = 0.2 × 2
(0.5 -0.1) x = 0.4 -0.2
0.4 x = 0.2
x = 0.5 L
0.5 L of A and 1.5 L of B should be mixed.
Example 35. Lowering of vapour pressure due to a solute in 1 molal aqueous solution at 100ºC is -
(a) 13.44 mm Hg
(b) 14.12 mm Hg
(c) 31.2 mm Hg
(d) 35.2 mm Hg
Solution.
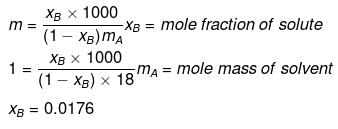
xA = 1 -0.0176 = 0.9824
p = p°xA
= 760 × 0.9824 = 746.62
Dp = p° - p = 760 -746.62
= 13.4
Example 36. The mass of a non-volatile solute (molecular mass = 40) which should be dissolved in 114 g octane to reduce its vapour pressure to 80% will be -
(a) 20 g
(b) 30 g
(c) 10 g
(d) 40 g
Ans. (c)
Solution.
If p° = 100, then p = 80
p = p° xA
80 = 100 × xA
xA = 0.80
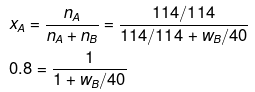
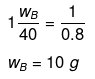
Example 37. A solution of urea in water has boiling point of 100.15ºC. Calculate the freezing point of the same solution if Kf and Kb for water are 1.87 K kg mol-1 and 0.52 K kg mol-1 respectively -
Solution.
ΔTb = (100.15 -100) = 0.15ºC
We know that, ΔTb = molality × Kb
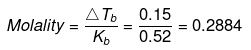
ΔTf = molality × Kf
= 0.2884 × 1.87 = 0.54ºC
Thus, the freezing point of the solution
= -0.54ºC.
Example 38. Calculate the molal depression constant of a solvent which has freezing point 16.6ºC and latent heat of fusion 180.75 J g-1 -
Solution.
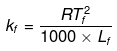
R = 8.314 J K-1 mol-1,
Tf = 16.6ºC = 273 16.6 = 289.6 K,
Lf = 180.75 J g-1
Substituting the values in the above equation,
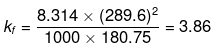
Example 39. The freezing point depression of 0.001 m Kx[Fe(CN)6 ] is 7.10 × 10-3 K. Determine the value of x. Given, Kf = 1.86 K kg mol-1 for water -
Solution. Dx = i × Kf × m
7.10 × 10-3 = i × 1.86 × 0.001
i = 3.817
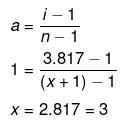
Molecular formula of the compound is
K3 [Fe(CN)6]
Example 40. A certain substance `A' tetramerises in water to the extent of 80%. A solution of 2.5 g of A in 100 g of water lowers the freezing point by 0.3ºC. The molar mass of A is -
(a) 122
(b) 31
(c) 244
(d) 62
Ans. (d)
Solution.
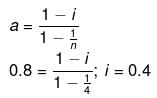
ΔT = iKf × m
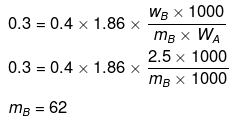
Example 41. van't Hoff factor of Hg2Cl2 in its aqueous solution will be (Hg2Cl2 is 80% ionized in the solution) -
(a) 1.6
(b) 2.6
(c) 3.6
(d) 4.6
Ans. (b)
Solution. 
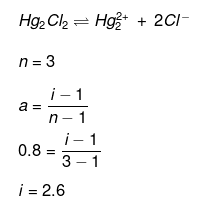
Example 42. Calculate the amount of NaCl which must be added to 100 g water so that freezing point is depressed by 2K. For water, Kf = 1.86 K kg mol-1 -
Solution. NaCl is a strong electrolyte. It is completely dissociated in solution.
Degree of dissociation, a = 1
NaCl  Na+1 Cl-1
Na+1 Cl-1
(n = 2)
No. of particles after dissociation = 1 (n -1) a
= 1 (2 -1) × 1 = 2
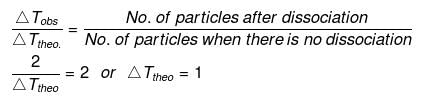
Let w g of NaCl be dissolved in 100 g of water.
So,
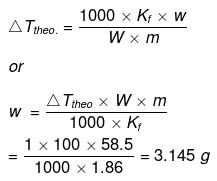
Example 43. The degree of dissociation of Ca(NO3)2 in a dilute solution containing 14 g of the salt per 200 g of water at 100ºC is 70%. If the vapour pressure of water is 760 mm, calculate the vapour pressure of solution -
Solution. Dptheo. = Lowering in vapour pressure when there is no dissociation
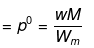
(given, p° = 760 mm, w = 14 g, W = 200 g, M = 18, m = 164)
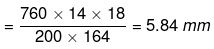
Degree of dissociation
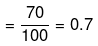
Ca(NO3)2 Ca2+ + 2NO3-
Ca2+ + 2NO3-
(n = 3)
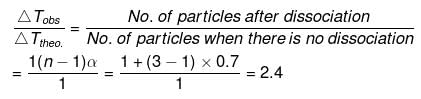
So, ΔTobs. = 2.4 × ΔTtheo. = 2.4 × 5.84
= 14.02 mm
p -ps = Dpobs. = 14.02
ps = ps -14.02 = 760 - 14.02 = 745.98 mm
Example 44. Calculate the normal boiling point of a sample of sea water found to contain 3.5% of NaCl and 0.13% of MgCl2 by mass. The normal boiling point of water is 100ºC and Kb (water) = 0.51 K kg mol-1. Assume that both the salts are completely ionised -
Solution.
Mass of NaCl = 3.5 g
No. of moles of NaCl 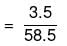
Number of ions furnished by one molecule of NaCl is 2.
So, actual number of moles of particles furnished by sodium chloride = 2 × Similarly, actual number of moles of particles furnished by magnesium chloride
= 3 × Total number of moles of particles
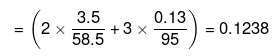
Mass of water = (100 -3.5 -0.13) = 96.37 g
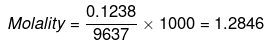
ΔTb = Molality × Kb
= 1.2846 × 0.51 = 0.655 K
Hence, boiling point of sea water = 373.655 K or 100.655ºC.
Example 45. Sea water is 3.5% by mass of a salt and has a density 1.04 g cm-3 at 293 K. Assuming the salt to be sodium chloride, calculate the osmotic pressure of sea water. Assume complete ionization of the salt.
Solution. Mass of NaCl = 3.5 g
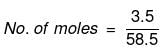
Actual number of moles of particles of solute in solution= 
Volume of solution = 1 litre
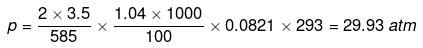
Example 46. Molality of a solution in aqueous medium is 0.8. Calculate its mole fraction and the percentage by mass of solute if molar mass of solute is 60.
Solution. We know that,
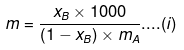
where, xB = mole fraction of solute
mA = molar mass of solvent
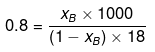
xB = 0.014
Let wB = x g, wA = 100 g
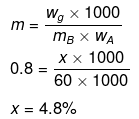
Example 47. A very small amount of a non-volatile solute (that does not dissociate) is dissolved in 56.8 cm3 of benzene (density 0.889 g cm-3). At room temperature, vapour pressure of this solution is 98.8 mm Hg while that of benzene is 100 mm Hg. Find the molality of the solution. If the freezing temperature of this solution is 0.73 degree lower than that of benzene, what is the value of molal freezing point depression constant of benzene?
Solution.
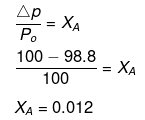
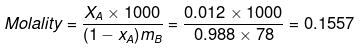
ΔTf = Kf × Molality
0.73 = Kf × 0.1557
Kf = 4.688
Example 48. x g of a non-electrolytic compound (molar mass = 200) is dissolved in 1.0 litre of 0.05 M NaCl solution. The osmotic pressure of this solution is found to be 4.92 atm at 27ºC. Calculate the value of `x'. Assume complete dissociation of NaCl and ideal behaviour of this solution.
(a) 16.52 gm
(b) 24.032 gm
(c) 19.959 gm
(d) 12.35 gm
Ans. (c)
Solution.
(i) For NaCl : p = iCRT = 2 × 0.05 × 0.0821 × 300 = 2.463 atm
(ii) For unknown compound,
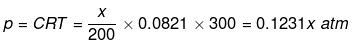
Total osmotic pressure p = p1 + p2
4.92 = 2.463 + 0.1231 x
x = 19.959 g
Example 49. The freezing point of a solution containing 50 cm3 of ethylene glycol in 50 g of water is found to be -34ºC. Assuming ideal behaviour, calculate the density of ethylene glycol (Kf for water = 1.86 K kg mol-1).
(a) 1.13 g/cm3
(b) 2.00 g/cm3
(c) 1.8 g/cm3
(d) 2.25 g/cm3
Ans. (a)
Solution.
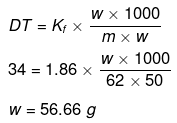
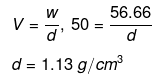
Example 50. Match the boiling point with Kb for x, y and z if molecular weight of x, y and z are same.
b. pt | Kb | |
x | 100 | 0.68 |
y | 27 | 0.53 |
z | 253 | 0.98 |
Solution. Molal elevation constant may be calculated as,
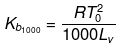
(where, Tºb = boiling point of pure solvent Lv = latent heat of vaporization.)
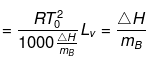
(here, ΔHV = molar latent heat of vaporization).
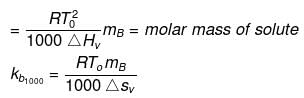
here, ΔSV = entropy of vaprization.
By considering ΔSV as almost constant, Kb µ Tº.
Kb(x) = 0.68 ; Kb (y) = 0.53 and Kb (z) = 0.98.
Example 51. 1.22 g C6H5COOH is added into two solvents and data of ΔTb and Kb are given as -
(a) ln 100 g CH3COCH3; ΔTb= 0.17; Kb = 1.7 kg kelvin/mol
(b) ln 100 g benzene; ΔTb = 0.13; Kb = 2.6 kg kelvin/mol
Find out the molecular weight of C6H5COOH in both cases and inerpret the result.
Solution.
(a)
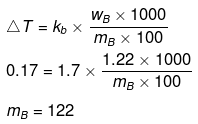
(b)
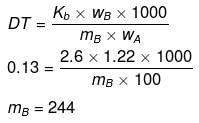
(Abnormally double molecular mass of benzoic acid, it shows association of benzoic acid in benzene).
Example 52. How much C2H5OH should be added to 1 litre H2O so that it will not freeze at -20ºC?
Kf = 1.86ºC/m
Solution. Mass of 1 litre water = 1000 g
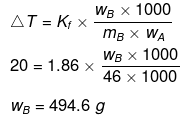
Example 53. Calculate the molarity of each of the ions in solution when 3.0 litre of 4.0 M NaCl and 4.0 litre of 2.0 M CoCl2 are mixed and diluted to 10 litre.
Solution. Molarity Na = 1.2 M
Molarity Co2+ = 0.8 M
Molarity Cl- = 2.8 M
Total Cl- ions = 28 mole.
Example 54. Calculate the molarity of each ion in solution after 2.0 litre of 3.0 M AgNO3 is mixed with 3.0 litre of 1.0 M BaCl2.
Solution.
BaCl2+ 2AgNO3 → 2AgCl +Ba(NO3)2
Initial 3 6 -_
mole
Final - -6 (ppt) 3
mole
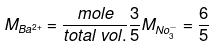
Example 55. 1.2 kg ethylene glycol  was added in a car radiator containing 9 litre water. The freezing of water was just prevented when car was running in the Himalayan valley at temperature -4ºC. Sudden thunderstorm in the valley lowered the temperature to -6ºC. Calculate the amount of ice separated.
was added in a car radiator containing 9 litre water. The freezing of water was just prevented when car was running in the Himalayan valley at temperature -4ºC. Sudden thunderstorm in the valley lowered the temperature to -6ºC. Calculate the amount of ice separated.
Solution.
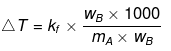
A → Solute; B → Solvent
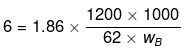
wB = 6000 g
weight of ice = (Total weight of H2O) - (wt. of H2O at 6°C) = 9000 - 6000 = 3000 g = 3 kg
Example 56. The vapour pressure of a solvent decreased by 10 mm of mercury when a non-volatile solute was added to the solvent. The mole fraction of the solute in the solution is 0.2. What should be the mole fraction of the solvent, if the decrease in the vapour pressure is to be 20 mm of mercury.
(a) 0.8
(b) 0.6
(c) 0.4
(d) 0.2
Ans. (b)
Solution.
Mole fraction of solute
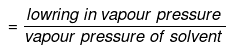
Comparing under the two conditions,
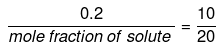
or mole fraction of solute = 0.4
mole fraction of solvent = (1 -0.4) = 0.6
Example 57. Three solutions of HCl having normality 12 N, 6 N and 2 N are mixed to obtain a solutions of 4 N normality. Which among the following volume ratio is correct for the above three components?
(a) 1 : 1 : 5
(b) 1 : 2 : 6
(c) 2 : 1 : 9
(d) 1 : 2 : 4
Ans. (b)
Solution.
Use Hit & Trial Method.
N1V1 + N2V2 + N3V3 = NR(V1 + V2 + V3)
12 × 1 6 × 2 2 × 6 = NR(9)
NR = 4
Example 58. Two solutions of H2SO4 of molarities x and y are mixed in the ratio of V1 mL : V2 mL to form a solution of molarity M1. If they are mixed in the ratio of V2mL : V1mL, they form a solution of molarity M2. Given V1. Given V1/V2 => 1 and  =
=  , then x : y is -
, then x : y is -
(a) 2 : 1
(b) 4 : 1
(c) 1 : 2
(d) 3 : 1
Ans. (a)
Solution.
Molarity of the mixture can be calculated as.
M1V1 + M2V2 = MR(V1 + V2)
where, MR = resultant solution
(V1 × x) × (V2 × y) = M1(V1 + V2)
(V2 × x) × (V1 × y) = M2(V1 + V2)
Dividing equation (i) by equation (ii), we get
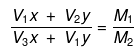
Substituting 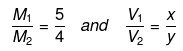 we can calculate x : y.
we can calculate x : y.
Example 59. Isuling (C2H10O5)n is dissolved in a suitable solvent and the osmotic pressure (p) of solutions of various concentrations (g/cc) C is measured at 20ºc. The slope of the plot of p against 'C' is found to be 4.65 × 10-3. The molecular weight of insulin is -
(a) 4.8 × 105
(b) 9 × 105
(c) 3 × 105
(d) 5.17 × 106
Ans. (d)
Solution.
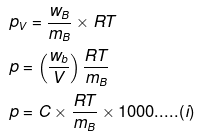
where C = concentration in g/cc,
Comparing eqs. (i) and (ii), ...(ii)

Example 60. Compound PdCl4.6H2O is a hydrated complex; 1 molal aqueous solution of it has freezing point 269.28 K. Assuming 100% ionization of complex, calculate the molecular formula of the complex (Kf for water = 1.86 K kg mol-1)
(a) [Pd(H2O)6]Cl4
(b) [Pd(H2O)4Cl2]Cl 2.2H2O
(c) [Pd(H2O)3Cl3]Cl.3H 2O
(d) [Pd(H2O)2Cl4].4H 2O
Ans. (c)
Solution.
ΔT = i × Kf × m
(273 -269.28) = i × 1.86 × 1
3.72 = i × 1.86
i = 2
Thus, the complex should give two ions in the solution, i.e., the complex will be [Pd(H2O)3Cl3]Cl.3H2O]
Example 61. pH of 0.1 M monobasic acid is measured to be 2. Its osmotic pressure at a given temperature T K is -
(a) 0.1 RT
(b) 0.11 RT
(c) 1.1 RT
(d) 0.01 RT
Ans. (b)
Solution.
HA  H+ A-
H+ A-
t = 0 C 0 0
teq C -C∝ C∝ C∝
[H ] = Ca, [H ] = 10-pH
Cα = 10-2
0.1 α = 10-2
a = 0.1
a =  ; 0.1 =
; 0.1 = 
i = 1.1
p = iCRT
= 1.1 × 0.1 × RT = 0.11 RT
Example 62. Lowering of vapour pressure in 1 molal aqueous solution at 100ºC is -
(a) 13.44 mm Hg
(b) 14.12 mm Hg
(c) 31.2 mm Hg
(d) 35.2 mm Hg
Ans. (a)
Solution.
Molality and mole fraction are related as follows:
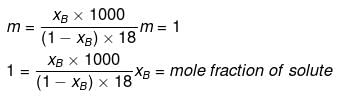
mA = molar mass of solvent
xB = 0.0176, xA = 0.9824
p = p°AxA
p = 760 × 0.9824 = 746.624
Δp = p°A - p = 760 - 746.624 = 13.4 mm Hg.
|
22 videos|162 docs|17 tests
|
FAQs on Solved Examples - Solutions (Part - 3) - Additional Study Material for JEE
| 1. What are some frequently asked questions about solving examples in JEE? |  |

|
Explore Courses for JEE exam
|

|

















