Short & Long Answer Questions: Haloalkanes & Haloarenes | Chemistry for JEE Main & Advanced PDF Download
Q.1 Which chlorine containing compound is used for treatment of typhoid fever?
Answer: Chloramphenicol is used for treatment of typhoid fever.
Q.2 Name the hormone, deficiency of which causes goitre.
Answer: Thyroxin deficiency causes goitre.
Q.3 In which disease chloroquine is used?
Answer: Chloroquine is used in malaria.
Q.4 What is the use of halothane?
Answer: Halothane is used as anesthetic during surgery.
HalothaneQ.5 Which type of halo compounds are used as blood substitute in surgery?
Answer: Fully fluorinated compounds are used as blood substitute.
Q.6 Name the hybridisation of carbon atom with which halogen atom is bonded in the following compounds. (i) Alkyl halide (ii) Allylic halide (in) Vinylic halide (iv) Aryl halide (v) Benzylic halides.
Answer: (i) sp3 (ii) sp3 (ii) sp2 (iv) sp2 (v) sp3
Q.7 Arrange the 1°, 2°, 3° alcohols in the order of reactivity towards given halo acids.
Answer: Increasing reactivity towards haloacids : 3° > 2° > 1°.
Q.8 What is the effect of branching on the boiling point of isomeric haloalkanes?
Answer: With increasing branching, boiling point decreases.
Q.9 Which class of organic reactions are common for alkyl halides?
Answer: Nucleophilic substitution is common for alkyl halides.
Q.10 What is ambient nucleophile?
Answer: Molecules or ions having two nucleophilic centres are called ambident nucleophiles.
Q.11 What is inversion of configuration?
Answer: Inversion of configuration means reversal of configuration of compound.
Q.12 What do you mean by chiral molecule?
Answer: The molecule which is non-super imposable on its mirror image is called chiral molecule.
Q.13 What is chirality?
Answer: The property of a molecule due to which it is non- super imposable on its mirror image is called chirality.
Q.14 What is asymmetric carbon?
Answer: The carbon which has all the four substituent's different are called asymmetric carbon.
Q.15 Define the term racemisation.
Answer: The equimolar mixture of d-form and l-form, which is optically inactive is known as racemic mixture and the process of its formation is called racemisation.
Q.16 What are enantiomers?
Answer: Optically active compounds which are mirror images of each other are called enantiomers.
Q.17 What are organometallic compounds?
Answer: Compounds containing carbon-metal bond are called organ metallic compounds e.g. RMgX.
Q.18 What is Grignard's reagent?
Answer: Alkyl magnesium halide is known as Grignard's reagen (RMgX).
Q.19 Write the full form of D.D.T.
Answer: Dichlorodiphenyltrichloro ethane.
Q.20 Name two haloalkanes responsible for depletion of ozone layer.
Answer: Freon and tetrachloromethane are responsible for depletion of ozone layer.
Q.21 Name two haloalkanes used as refrigerants.
Answer: Freon and chloroform are used as refrigerants.
Q.22 What is halo form?
Answer: Trihalogen derivatives of methane are called haloform. e.g. CHCl chloroform.
Q.23 Which is better nucleophile, a bromide ion or an iodide ion?
Answer: An iodide ion.
Q.24 What is the hybridisation of carbon of benzne bonded to halogen in aryl halide?
Answer: sp2.
Q.25 How are ortho and para isomers of aryl halides separated from each other?
Answer: Due to large difference in their melting points they are separated by fractional distillation method.
Q.26 The boiling point of alkyl halides are higher than those, the hydrocarbons of comparable molecular mass. Explain.
Answer: Molecules of organic halogen compounds are generally polar. Due to greater polarity as well as higher molecular mass as compared to parent hydrocarbon, the intermolecular forces of attraction (dipole-dipole interaction and Van der Waals force) are stronger and have higher boiling point than hydrocarbons of comparable molecular masses.
Q.27 Why the boiling points of isomeric halo alkanes decrease with increasing branching?
Answer: As the branching increases molecule tends to acquire spherical shape i.e. its surface area decreases, correspondingly the magnitude of van der Waals force also decreases. So, boiling point also decreases.
Q.28 Haloalkanes are only slightly soluble in water. Why?
Answer: Alkyl halides though polar due to presence of electronegative halogen atom are insoluble in water since they do not form hydrogen bonds. The energy required to break intermolecular H-bonds of water is much higher than the energy released by interaction between water and halide molecules.
Q.29 Haloalkanes are more soluble in organic solvents. Why ?
Answer: Haloalkanes tend to dissolve in organic solvent because new intermolecular attraction between haloalkanes and solvent molecules have much the same strength as the ones being broken in separating haloalkane and solvent molecules.
Q.30 The use of DDT is banned in some countries, why?
Answer: Many countries banned the use of DDT due to following reasons: (i) Many insects developed resistance to DDT and it was also found to have high toxicity towards fish. (ii) DDT is not metabolised very rapidly by animals, instead it is deposited and stored in fatty tissues.
Q.31 What are the disadvantages of using freons?
Answer: In stratosphere, freons are able to initiate radical chain reactions that can upset the natural ozone balance.
Q.32 Why is iodoform antiseptic?
Answer: landform behaves as antiseptic due to liberation of free iodine.
Q.33 Name three halogen containing organic compounds used in medicine.
Answer: Chloramphenicol, chloroquine, halothane.
Q.34 Aryl halides cannot be prepared by the reaction of hydrogen halide on phenol. Why?
Answer: Due to resonance, in phenol molecule C-O bond has some double bond character due to which it is difficult to break it. Hence, aryl halide cannot be prepared by reaction of hydrogen halide on phenol.
Q.35 Why ortho andpara halo toluene can be separated easily?
Answer: Due to their large difference in boiling points, they can be separated easily by fractional distillation.
Q.36 Reactions of arene with iodine is carried out in the presence of an oxidising agent. Why?
Answer: Reactions of arene with iodine are reversible in nature and require the presence of an oxidising agent to oxidise the HI formed during the reaction and shift the reaction towards right.
Q.37 Explain why no effect on reactivity of haloarene is observed by the presence of electron withdrawing group at meta position?
Answer: The presence of electron withdrawing group increases the reactivity of haloarenes. The effect is more pronounced at ortho and para position. From resonance structure of chlorobenzene we see that electron density is maximum only at o/p-position. So, it does not affect much at w-position.
Q.38 Electrophilic substitution reactions in haloarenes occur slowly and require more drastic conditions.
Answer: Due to ?I effect halogen withdraws electron towards it and overall deactivation takes place and for better yield drastic conditions are required.
Q.39 Chlorine is electron withdrawing but ortho, para directing in electrophilic aromatic substitution reactions. Why?
Answer: Even though chlorine is electron withdrawing the electron density is maximum at ortho and para positions.
Q.40 Classify the following as alkyl, alkyl and vinyl halides (i) H2CH=CFCH2CH3 (ii) (CH3)2CClCH3 (iii) CH2=CHCH2I (iv) 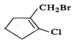
Answer: (i) Vinyl fluoride (ii) Alkyl chloride (iii) Allyl iodide (iv) Vinyl chloride and alkyl bromide.
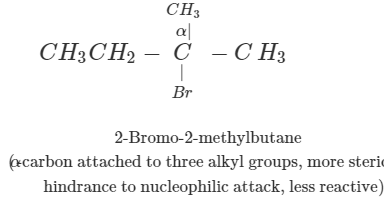
Q.41 What is the decreasing order of reactivity of the following in SN2 reaction? 1-Bromo-2-methylbutane, 1-Bromo-2-2-dimethylpropane, 1-Bromopentane.
Answer: All are primary alkyl halides and their structural formulae are: 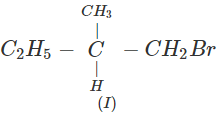
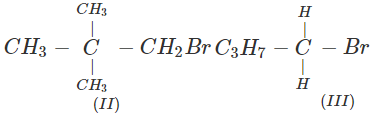 The order of reactivity is: (III) > (I) > (II) Explanation: The SN2 reactions are sensitive to steric hindrance. Greater the steric hindrance to the attacking nucleophile, lesser will be the reactivity. In the light of this, the reactivity order is justified.
The order of reactivity is: (III) > (I) > (II) Explanation: The SN2 reactions are sensitive to steric hindrance. Greater the steric hindrance to the attacking nucleophile, lesser will be the reactivity. In the light of this, the reactivity order is justified.
Q.42 What happens when chlorine is passed through boiling toluene?
Answer: The hydrogen atoms of the −CH3 group are replaced one after the other with chlorine atoms.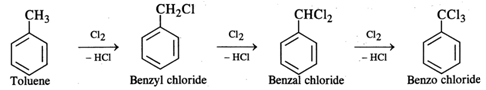
Q.43 What is the difference between hexachlorobenzene and benzene hexachloride?
Answer: Hexachlorobenzene is a substituted compound obtained by replacing all the hydrogen atoms in benzene with chlorineatoms. On the other hand, benzene hexachloride is an addition compound formed when three moles of chlorine add on one mole of benzene under reaction conditions.
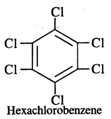
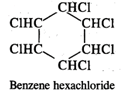
Q.44 Out of Br− and I− ions which is a better nucleophile?
Answer: Iodide ion (I−) is a better nucleophile than bromide ion (Br−) because iodine is less electronegative and therefore, electron pair can be more readily released by I− ion than by Br− ion.
Q.45 Arrange the following halides in order of increasing SN2 reactivity: H3Cl,CH3Br,CH3CH2Cl2,(CH3)2CHCl.
Answer: The reactivity of alkyl halides towards SN2 reaction decreases (i) with the increase in the size of alkyl group (ii) in the order C−I>C−Br>C−Cl. Therefore, the increasing order of the reactivity of the listed alkyl halides is: (CH3)2CHCl<CH3CH2Cl<CH3Cl<CH3Br.
Q.46 The reaction of primary alkyl halide with nitrite salt produces both RNO2 and RONO. Account for this behaviour.
Answer: Nitrite ion is an ambident nucleophile [O−−N=O]. Therefore, it can attack as nucleophile through both oxygen andnitrogen atoms and leads to the formation of two compounds.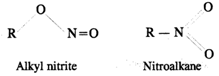
Q.47 The hydrocarbon styrene (C6H5CH=CH2) can be prepared by the dehydrohalogenation of either 1-bromo-2-phenylethane or 1-bromo-1-phenylethane using alcoholic KOH. Which alkyl halide will take part in the reaction?
Answer: 1-bromo-1-phenylethane is a secondary alkyl halide while the other is of primary nature. The alkene is expected to be formed by E2 elimination reaction. In this reaction, secondary alkyl halide is expected to give the desired alkene.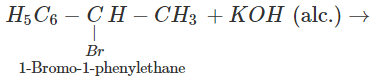
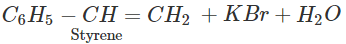
Q.48 Among the aromatic compounds with molecular formula C7H7Cl, how many isomers are possible? Which of these is maximum reactive in nature?
Answer: Four isomers are possible as shown below: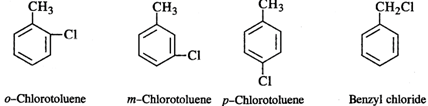 Out of these, the cleavage of C−Cl bond can easily take place in benzyl chloride to form a benzyl carbocation that is resonance stabilised. Therefore, benzyl chloride is maximum reactive in nature.
Out of these, the cleavage of C−Cl bond can easily take place in benzyl chloride to form a benzyl carbocation that is resonance stabilised. Therefore, benzyl chloride is maximum reactive in nature.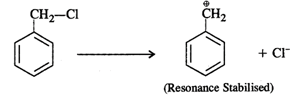
Q.49 Why is halogen atom attached to benzene ring ortho and para directing but deactivating in nature?
Answer: The lone electron pairs on the halogen atom are in conjugation or resonance with the pi-electron pairs of the ring (+ M effect). As a result, the ortho and para positions in the ring are the points of high electron density or negatively charged. The electrophilic substitution takes place at these positions. However, due to -Ieffect, the halogen atom deactivates the ring to some extent. For more details, consult Section 11.14.
Q.50 A compound is formed by the substitution of two chlorine atoms by two hydrogen atoms in propane. What is the number of structural isomers possible?
Answer: Four structural isomers are possible. These are: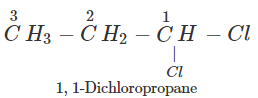
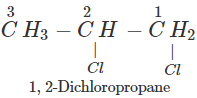
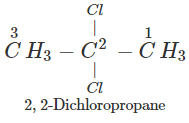
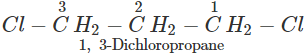
Q.51 Which compound in the following pairs would react faster in SN2 displacement reactions? 1-Bromopentane or 2-Bromopentane. 1-Bromo-2-methylbutane or 2-Bromo-2-methylbutane.
Answer: 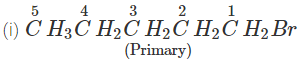
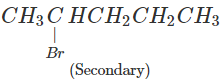
1-Bromopentane would react faster since it is a primary alkyl halide and moreover there is less steric hindrance experienced by the attacking nucleophile.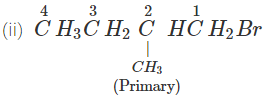
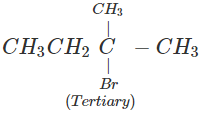 1-Bromo-2-methylbutane would react faster since it is a primary alkyl halide while the other is of tertiary nature.
1-Bromo-2-methylbutane would react faster since it is a primary alkyl halide while the other is of tertiary nature.
Q.52 Arrange the compounds CH3F,CH3I,CH3Br,CH3Cl in order of increasing reactivity in bimolecular nucleophilic substitution (SN2) reactions.
Answer: CH3F<CH3Cl<CH3Br<CH3I In these nucleophilic substitution reactions, the nucleophile is to displace the halide ion (X−). Greater the bond dissociation enthalpy of the C-X bond, difficult is its cleavage and lesser will be the reactivity. The order of bond dissociation enthalpy of different C-X bonds is: C−F>C−Cl>C−Br>C−I The order of reactivity towards SN2 reactions is the reverse.
Q.53 Predict the product of the following reaction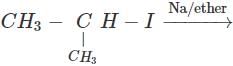
Answer: 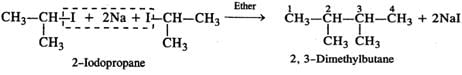
Q.54 What happens when benzal chloride is boiled with aqueous NaOH solution?
Answer: Benzaldehyde is formed. 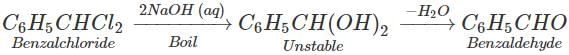
Q.55 Alkyl chlorides can be prepared by refluxing alcohol with thionyl chloride in the presence of pyridine. Why is pyridine used?
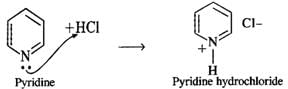
Answer: Thionyl chloride is the chlorinating agent in the reaction. 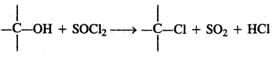 Pyridine is a tertiary base and forms a salt with HCl. Thus, it removes one of the products and facilitates the above reaction in the forward direction.
Pyridine is a tertiary base and forms a salt with HCl. Thus, it removes one of the products and facilitates the above reaction in the forward direction.
Q.56 Why is cyclopropyl chloride less reactive than cyclopentyl chloride towards SN1 reaction?
Answer: In SN1 reaction, a carbocation intermediate is formed in the slow or rate determining step by the cleavage of C−Cl bond. Now, the cyclopropyl carbocation intermediate is less stable than cyclopentyl carbocation due to greater strain. Therefore, cyclopropyl chloride is less reactive than cyclopentyl chloride towards SN1 reaction.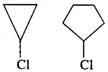
Q.57 When heated to 300°C, neopentyl chloride forms 2-chloro-2-methylbutane. Why?
Answer: Upon heating strongly, neopentyl chloride undergoes cleavage of C−Cl bond to form a primary carbocation which rearranges to form a more stable tertiary carbocation.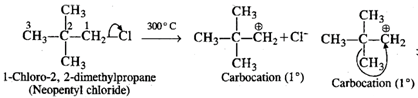
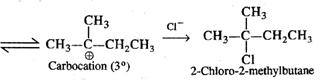
Q.58 Name the alkyl halide which can be used to prepare methane and ethane in single step.
Answer: Methyl halide gives methane by reduction and ethane as a result of Wurtz reaction.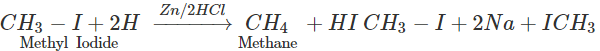
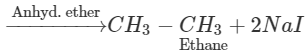
Q.59 What happens when benzene is reacted with chlorine in the presence of ultraviolet light?
Answer: Benzene hexachloride is formed as the addition product.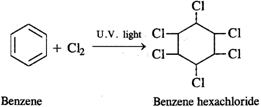
Q.60 Perfluorocarbons are remarkably stable; why?
Answer: The prefix per implies that all the hydrogen atoms of a particular hydrocarbon have been replaced by F atoms. For example, CF3(CF2)6CF3 or CF3−CF2−CF2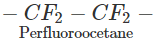 CF2−CF2−CF3 Since F atom is strongly electronegative and has a very high negative electron gain enthalpy, C- F bond is of polar nature. With the increase in the number of F atoms around the carbon atom, the magnitude of +δ charge on it increases and it acquires more electrostatic force of attraction for δ-charge on F atoms. As a result, strength of C- F bond increases and perfluoro carbons become quite stable as well as inert.
CF2−CF2−CF3 Since F atom is strongly electronegative and has a very high negative electron gain enthalpy, C- F bond is of polar nature. With the increase in the number of F atoms around the carbon atom, the magnitude of +δ charge on it increases and it acquires more electrostatic force of attraction for δ-charge on F atoms. As a result, strength of C- F bond increases and perfluoro carbons become quite stable as well as inert.
Q.61 Identify the compound in the following pair that reacts with sodium iodide in acetone at a faster rate towards SN2 mechanism. 1-Chlorohexane or cyclohexyl chloride:
Answer:
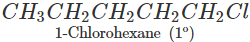
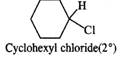 1-Chlorohexane is a primary alkyl halide and is expected to be more reactive towards SN2 reactions as compared to cyclohexyl chloride which is secondary in nature. This is because of less steric hindrance present in primary alkyl halide.
1-Chlorohexane is a primary alkyl halide and is expected to be more reactive towards SN2 reactions as compared to cyclohexyl chloride which is secondary in nature. This is because of less steric hindrance present in primary alkyl halide.
Q.62 Give the major products when 2-Bromo 3-methylbutane is reacted with sodium ethoxide.
Answer: Two products are formed in this reaction 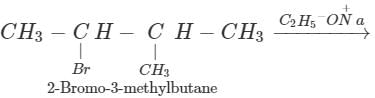
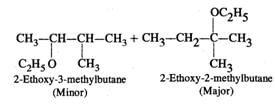 Mechanism:
Mechanism: 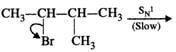
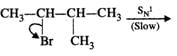
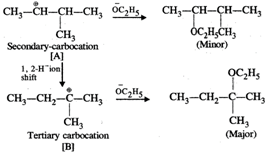 Please note that the tetiary carbocation formed as a result of 1, 2-hydride ion shift is more stable than the secondary carbocation.
Please note that the tetiary carbocation formed as a result of 1, 2-hydride ion shift is more stable than the secondary carbocation.
Q.63 Three alkyl halides A, B and C with molecular formula C7H15Cl have different boiling points. These are optically active in nature. On reacting with Mg dissolved in ether and then with water, each compound gives 2, 4-dimethylpentane. Suggest structures for the three isomers.
Answer: The structure of hydrocarbon is:
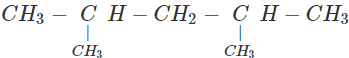
The possible structures of three isomeric haloalkanes [A], [B] and [C] are: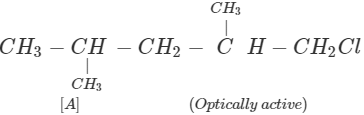
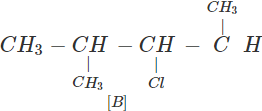
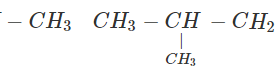
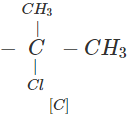
Q.64 Why does not ammonolysis of alkyl halides yield pure amines?
Answer: Alkyl halides react with ammonia to form a mixture of primary, secondary, tertiary amines and quaternary ammonium salts. Therefore, pure amines are not obtained as a result of the reaction. For details, consult Section 11.7.
Q.65 2-Bromopentane is treated with alcoholic KOH solution. What is the major product formed in this reaction? What is the name of the reaction?
Answer: The reaction is known as β-elimination and the major product is pent-2-ene according to Saytzeff's rule.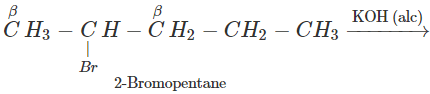
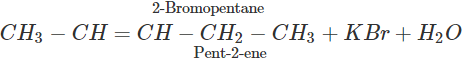
Q.67 Explain why displacement of cyanide ion and amide ion is not observed in nucleophilic substitution reaction.
Answer: Both cyanide ion (CN−) and amide ion (NH−2) are powerful nucleophiles and it is not easy to displace them by other nucleophiles in the nucleophilic substitution reactions.
Q.68 Why is chloroform not used as anaesthetic these days? What is the commonly used anaesthetic?
Answer: Chloroform is not used as an aesthetic because of its slow oxidation to poisonous gas phosgene. Halothane (CF3CHClBr)is the commonly used an aesthetic these days.
Q.69 Which compound in the following pairs will react faster in SN2 reaction? (a) CH3BrorCH3I (b) (CH3)3Br or CH3Br (c) CH2=CHBror CH2=CH−CH2Br.
Answer: (a) CH3Iwill react faster since C−I bond can cleave more easily as compared to C?Br bond. (b) CH3Brwill react faster because less steric hindrance as compared to (CH3)3CBr. (c) CH2CH−CH2Br will react faster because in CH2=CHBr molecule, C−Br bond has a partial double bond character due to resonance and its cleavage is more difficult as compared to CH2=CH−CH2−Br in which the bond does not have partial double bond character.
Q.70 Explain the formation of the products in the following reaction. CH3CH=CHCH2Br+H2O→CH3CH=CHCH2OH+CH3CH(OH)CH=CH2
Answer: This is a nucleophilic substitution reaction. The carbocation initially formed is resonance stabilised and gives two different alcohols on reacting with water.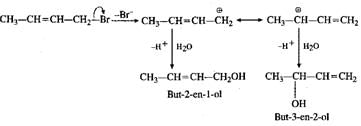
Q.71 Arrange the following compounds in increasing order of SN1 reactivity.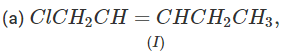
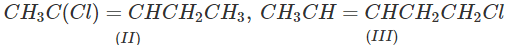
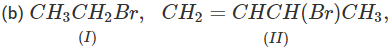
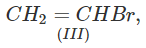
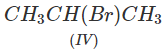
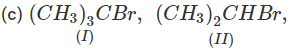
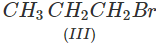
Answer: The order of reactivity of alkyl halides towards SN1 reactions is : Allylic > Tertiary > Secondary > Primary > Vinylic Based on this, the increasing order of reactivity in different alkyl halides listed above is : (a) (II) < (III) < (I) (b) (III) < (I) < (IV) < (II) (c) (III) < (II) < (I).
Q.72 Explain why reaction of HCl with CF2CH=CH3 proceeds according to Antimarkownikov's rule.
Answer: Fluorine (F) being a highly electronegative element has a strong -Ieffect. Therefore, when H+ from acid (electrophile) attacks the substrate, a primary carbocation is preferably formed. This reacts with the anion to give the desired product.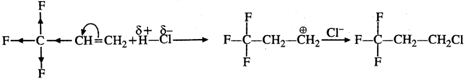
Q.73 Predict the order of reactivity of following compounds in SN1 reactions.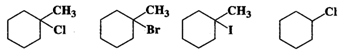
Answer: Except for chlorocyclohexane which is a secondary halide, the other compounds are tertiary halides in nature. We know that tertiary halides are more reactive than secondary halides in SN1 reactions. Moreover, the order of the cleavage of C−X bonds is: C−I>C−Br>C−Cl. Based on these guidelines the increasing order of reactivity of the cyclic compounds listed above is: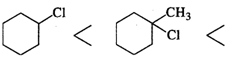
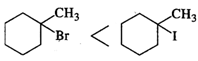
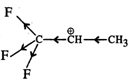
Q.74 Out of 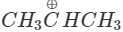 and
and 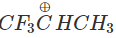 which is more reactive and why?
which is more reactive and why?
Answer: 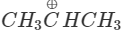 more reactive because F atom being highly electronegative has a strong-I effect. It destabilises the carbocation towards nucleophilic attack as compared to
more reactive because F atom being highly electronegative has a strong-I effect. It destabilises the carbocation towards nucleophilic attack as compared to 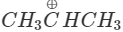 in which CH3 group is an electron releasing group and it stabilises the carbocation.
in which CH3 group is an electron releasing group and it stabilises the carbocation.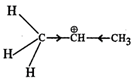
Q.75 What will be the major organic product of the following reaction? C6H5C2H5 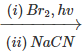
Answer: When ethyl benzene is treated with in presence of light, the bromine free radical formed during the reaction, abstracts the α (but not β-)hydrogen from the ethyl group to form a resonance stabilized benzylic radical (I) which then reacts with Br2 to form 1-bromo-1-phenylethane(II). This on treatment with NaCN undergoes nucleophilic substitution to form 2-phenylpropanenitrile (III).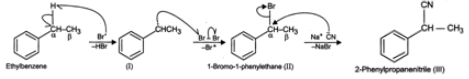
Q.76 Write down the structure of the product of the following reactions. 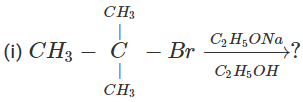 (ii) 3-Methyl-1-butene
(ii) 3-Methyl-1-butene  ?
?
Answer: (i) 3∘Alkyl halides on treatment with a strong base undergo dehydrohalogenation to form alkenes, i.e., 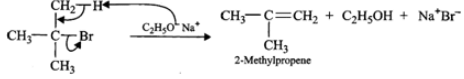
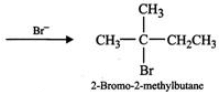
(ii) Addition of H+ to 3-methyl-1-butene first gives 2∘ carbocation (I) which being less stable rearranges to the more stable 3∘ carbocation (II). Nucleophilic attack by Br− ion on this carbocation gives 2-bromo-2-methylbutane as the major product,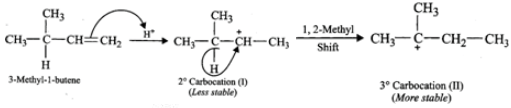
Q.77 An alkyl halide (A), on reaction with magnesium in dry ether followed by treatment with ethanol gave 2-methylbutane. Write all the possible structures of A.
Answer: The structures of all the possible alkyl halides that can be obtained by monohalogenation of 2-methylbutane are :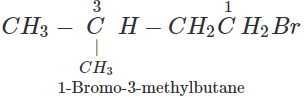
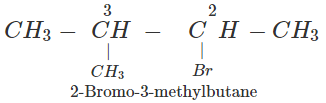 z
z
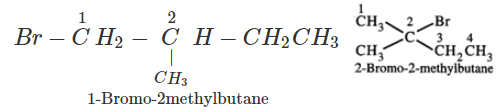
The Grignard reagents of all these four alkyl halides will give 2-methylbutane on treatment with ethanol.
Q.78 Differentiate between chiral and achiral molecules.
Answer: A chiral molecule is one that is not super imposable on its mirror image. These molecules contain one asymmetric or chiral carbon atom. For example, butan-2-ol. An achiral molecule, on the other hand, is one which is super imposable on its mirror image. For example, propan-2-ol.
Q.79 Identify and indicate the presence of centre of chirality, if any, in the following molecules? How many stereo isomers are possible for each. (i) 2-Aminobutane (ii) 3-Bromopent-1-ene (iii) 1, 2-Dichloropropane. (iv) 3-Methyl-1-pentene
Answer:
(i)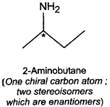 (ii)
(ii)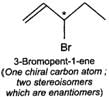 (iii)
(iii)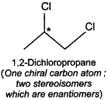 (iv)
(iv)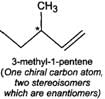
Q.80 What are enantiomers? Draw the structures of the possible enantiomers of 3-methylpent-1-ene.
Answer: Stereo isomers which are non-super imposable mirror images of each other are called enantiomers. The enantiomers of 3-methylpent-1-ene are : 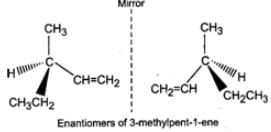
Q.81 Differentiate between retention and inversion.
Answer: If the relative configuration of the atoms/groups around a chiral centre in an optically active molecule remains the same before and after the reaction, the reaction is said to proceed with retention of configuration. On the other hand, if the relative configuration of the atoms/groups around a chiral centre in the product is opposite to that in the reactant, the reaction is said to proceed with inversion of configuration. For example,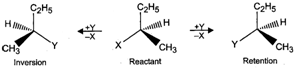
Q.82 Arrange the following halides in order of increasing SN2 reactivity : CH3Cl,CH3Br,CH3CH2Cl,(CH3)2CHCl.
Answer: As the size of the alkyl group increases, SN2 reactivity decreases. Further, C−Br bond being weaker is easier to break than C−Cl bond. Therefore, the overall increasing SN2 reactivity follows the order : (CH3)2CHCl<CH3CH2Cl<CH3Cl<CH3Br.
Q.83 Predict the order of reactivity of the following compounds in SN1 reactions.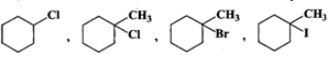
Answer: The first compound is a 2∘ alkyl halide while all others are 3∘ alkyl halides. Since 3∘ alkyl halides are more reactive than 2∘ alkyl halides in SN1 reactions, therefore, first compound is the least reactive. Further reactivity increases in the order: chloride < bromide < iodide. Thus, the increasing order of reactivity in SN1 reactions is the same in which they are listed above.
Q.84 Which one of the following pairs undergo SN1 substitution reaction faster and why? (i) 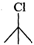 or
or 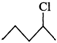 (ii)
(ii) 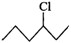 or
or 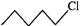
Answer: The reactivity in SN1 reactions depends upon the stability of the intermediate carbocation which an alkyl halide gives on ionization. (i) 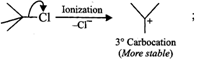
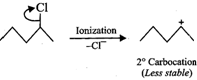 Since 30 carbocations are more stable than 20 carbocations, therefore,
Since 30 carbocations are more stable than 20 carbocations, therefore, 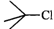 will react faster than
will react faster than 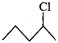 (ii)
(ii) 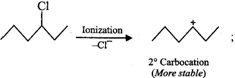 Since 20 carbocations are more stable than 10 arbocations, therefore,
Since 20 carbocations are more stable than 10 arbocations, therefore, 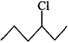 reacts faster than
reacts faster than  .
.
Q.85 Which compound in the following couples, will react faster in SN2 displacement and why? (a) 1-Bromopentane or 2-bromopentane (b) 1-Bromo-2-methylbutane or 2-bromo-2-methylbutane.
Answer: In SN2 reactions, reactivity depends upon the steric hindrance. (a) In 2-bromopentane, the α-carbon atom attached to Br atom is further linked to two alkyl groups, i.e., methyl and n-propyl but in 1-bromopentane, it is attached to only one alkyl group, i.e., n-butyl group. Therefore, 2-bromopentane suffers greater steric hindrance to nucleophilic attack than 1-bromopentane and hence 1-bromopentane is more reactive than 2-bromopentane in SN2 reactions.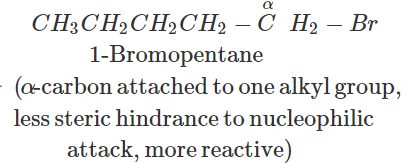

(b) In 1-bromo-2-methylbutane, α-carbon is attached to one bulky sec. -butyl group while in 2-bromo-2-methylbutane,α-carbon is attached to three alkyl groups, i.e., two methyl and one ethyl. Therefore, 2-bromo-2-methylbutane suffers greater steric hindrance to nucleophilic attack than 1-bromo-2-methylbutane, and hence 1-bromo-2-methylbutane is more reactive than-2-bromo-2-methylbutane. 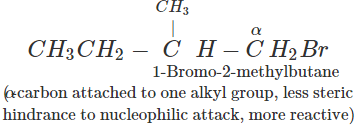
Q.86 The following reaction gives two products.
C6H5CH2CHClC6H5  Write the structures of the products.
Write the structures of the products.
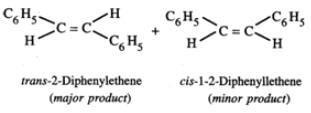
Answer: C6H5CH2CHClC6H5 upon heating with alcoholic KOH, undergoes dehydrochlorination to yield 1, 2-diphenylethene (stilbene) which exhibits geometrical isomerism. Thus, the two products formed are : trans-1, 2-diphenylethene and cis-1, 2-diphenylethene. 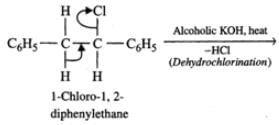
Q.87 Account for the following: (a) Chloromethane reacts with KCN to form ethanitrile as main product and with AgCN to form methyl isocyanide as chief product. (b) Use of DDT was banned in United States in 1973. (c) Benzylic halides show high reactivity towards SN1 reaction.
Answer: (a) Cyanide ion is an ambident ion. It has the following two resonating structures: 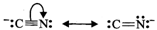 Since KCN is predominantly ionic, the reaction can occur either through carbon or nitrogen. Because C-C bonds are stronger than C-N bonds, therefore, carbon end of CN- ion acts as the nucleophile on chloromethane to give enthanenitrite as the main product.
Since KCN is predominantly ionic, the reaction can occur either through carbon or nitrogen. Because C-C bonds are stronger than C-N bonds, therefore, carbon end of CN- ion acts as the nucleophile on chloromethane to give enthanenitrite as the main product. 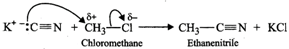 In contrast, Ag?CN: is predominantly covalent. Therefore, only electron pair on nitrogen is available for nucleophilic attack on chloromethane to form methyl isocyanide as the chief product
In contrast, Ag?CN: is predominantly covalent. Therefore, only electron pair on nitrogen is available for nucleophilic attack on chloromethane to form methyl isocyanide as the chief product 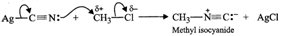 (b) Due to chemical stability of DDT and its fat solubility, DDT is not completely metabolized very rapidly by the animals. Instead it gets deposited and stored in fatty tissues of animals which causes toxicity. As a result, use of DDT was banned in U.S. in 1973. (c) Benzyl halides reality undergo ionization to give benzyl cation which is stabilized by resonance (refer to page 10/31). Since carbocations are reactive chemical species, therefore, benzyl halides are very reactive in SN1 reactions.
(b) Due to chemical stability of DDT and its fat solubility, DDT is not completely metabolized very rapidly by the animals. Instead it gets deposited and stored in fatty tissues of animals which causes toxicity. As a result, use of DDT was banned in U.S. in 1973. (c) Benzyl halides reality undergo ionization to give benzyl cation which is stabilized by resonance (refer to page 10/31). Since carbocations are reactive chemical species, therefore, benzyl halides are very reactive in SN1 reactions.
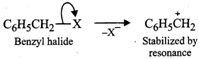
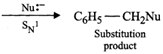
Q.88 Propose a mechanism of the reaction taking place when (a) (-)-2-Bromooctane reacts with sodium hydroxide to form (+)-octan-2-ol (b) 2-Bromopentane is heated with (ale). KOH to form alkenes.
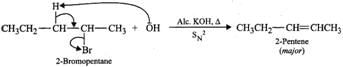
Answer: (a) 2-Bromooctane is a chiral molecule and exists as two optical isomers (enantiomers), i.e., (+) and (-). 2-bromooctane. When (-)-2-bromooctanes is treated with NaOH, it undergoes SN2 reaction in which OH−ion attacks from the backside. Inversion of configuration occurs and (+)-octan-2-ol is formed (refer to Fig. 10.10, page 10/36). (b) 2-Bromopentane on heating with alc. KOH undergoes dehydrohalogenation to give a mixture of two alkenes, i.e., 2-pentene as the major product (Sayzeff product) and 1-pentene as the minor product.
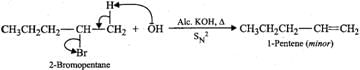
Q.89. C6H5CHClCH3 is hydrolysed more easily with KOH than C6H5CH2Cl . Explain.
Answer: Both C6H5CHClCH3and C6H5CH2Clare benzylic halides and hence undergo hydrolysis in aqueous KOH by SN1 mechanism. But in SN1 reactions,
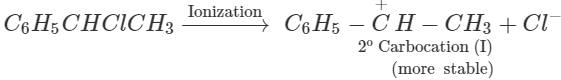
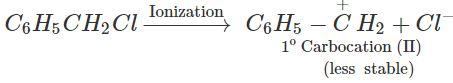
reactivity depends upon the stability of the intermediate carbocations. Now C6H5−CHClCH3 on ionization gives a 2∘ carbocation, C6H5C+HCH3(I) which is stabilized both by + R-effect of the C6H5 group and +1-effect of the CH3 group but C6H5CH2Cl on ionization gives a 1∘ carbocation C6H5C+H2(II) which is only stabilized by +R-effect of the C6H5C+H2. Since carbocation (I) is more stable than carbocation(II), therefore, C6H5CHClCH3 undergoes hydrolysis more easily than C6H5CH2Cl.
Q.90 Arrange the following in the decreasing order of reactivity towards SN2 displacement reaction and give reasons in support of your answer. (a)C2H5Br,C2H5I,C2H5Cl (b) (CH3)3CBr,CH3CH2CHBrCH3,CH3CH2CH2CH2Br
Answer: (a) The reactivity increases as the carbon-halogen bond dissociation enthalpy decreases. Since the bond dissociation enthalpy decreases in the order: C−Cl>C−Br>C−I, therefore, reactivity of the three ethyl halides decreases in the reverse order, i.e., C2H5I>C2H5Br>C2H5Cl (b) In SN2 reactions, reactivity decreases as steric hindrance around the carbon atom carrying the halogen increases. Now in (CH3)3CBr, there are three bulky CH3 groups at the carbon carrying Br, in CH3CH2CHBrCH3, there two bulky alkyl groups ; one CH3 and one CH3CH2 at the carbon carrying Br but in CH3CH2CH2CH2Br there is only one bulky CH3CH2CH2 group and two small H atoms. As a result, steric hindrance increases in the order : CH3CH2CH2CH2Br<CH3CH2CHBrCH3<(CH3)3CBr. Therefore, reactivity decreases in the reverse order i.e., CH3CH2CH2CH2Br>CH3CH2CHBrCH3>(CH3)3CBr.
Q.91 CHF3 is less acidic than CHCl3. Explain.
Answer: Due to stronger -I-effect of F than Cl,CHF3 should be more acidic than CHCl3. But actually reverse is true. This is due to the reason that : CCl−3 left after the removal of a proton from CHCl3 is stabilized by resonance due to the presence of d-orbitals on Cl but: CF−3 left after the removal of a proton from CHF3 is not stabilized by resonance due to the absence of d-orbitals on F.
Q.92 Complete the following reaction. CH3−CH=CH2 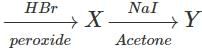
Answer: 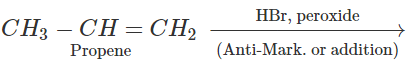
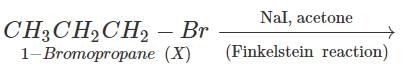
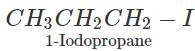
Q.93 Arrange the following compounds in order of increasing boiling points. 1-Chlorobutane, isopropyl chloride, 1-Chlorobutane
Answer: (i) For the sample halogen atom, the boiling points of 1∘ alkyl halides with the increase in the number of carbon atoms in the alkyl chain due a corresponding increase in the surface area and hence van der Waals forces of attraction. Thus b.p. of 1-Chlorobutane (351.5 K) is higher than 1-Chloropropane (320 K). (ii) For the same halogen atom, the b.p. of 2∘ alkyl halides is lower than the corresponding 1 alkyl halide due to a corresponding decrease in surface area and hence van der Waals forces of attraction. Thus, b.p. of isopropyl chloride (309 K) is lower than 1-Chloropropane (320 K). Thus, the b.p. of the three alkyl halides increase in the order : Isopropyl chloride < 1-Chloropropane < 1-Chlorobutane
|
334 videos|656 docs|300 tests
|
FAQs on Short & Long Answer Questions: Haloalkanes & Haloarenes - Chemistry for JEE Main & Advanced
| 1. What are haloalkanes and haloarenes? |  |
| 2. What are the uses of haloalkanes and haloarenes? |  |
| 3. How are haloalkanes and haloarenes named? |  |
| 4. What are the physical properties of haloalkanes and haloarenes? |  |
| 5. What are the environmental concerns associated with haloalkanes and haloarenes? |  |

















