NCERT Solutions for Class 8 Science Chapter 4 - Combustion and Flame
Exercises
Q1. List conditions under which combustion can take place.
Ans: The conditions required for combustion to take place are:
- Presence of a fuel.
- Air (or oxygen).
- Ignition temperature (minimum temperature at which a substance catches fire).
Q2. Fill in the blanks:
(a) Burning of wood and coal causes …………….of air.
Ans: Pollution
(b) A liquid fuel, used in homes is …………….
Ans: LPG
(c) Fuel must be heated to its ………… …………….before it starts burning.
Ans: Ignition temperature
(d) Fire produced by oil cannot be controlled by …………….
Ans: Water
Q3. Explain how the use of CNG in automobiles has reduced pollution in our cities.
Ans:
- Combustion of fuels like petroleum causes formation of un-burnt carbon particles along with carbon monoxide gas. These harmful pollutants enter the air and cause respiratory diseases.
- Compressed Natural Gas (CNG) produces these harmful products in very less quantity. It is a comparatively cleaner fuel. Therefore, the use of CNG has reduced pollution in our cities.
Q4. Compare LPG and wood as fuels.
Ans: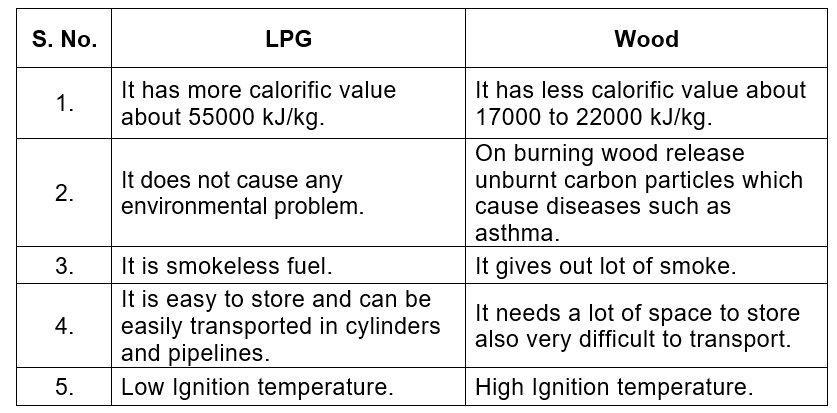
Q5. Give reasons:
(a) Water is not used to control fires involving electrical equipment.
Ans: Water is a conductor of electricity, so it can easily conduct electric current and cause danger of electric shocks or short-circuits. Therefore, water can not be used to control the fire involving electrical equipment.
(b) LPG is a better domestic fuel than wood.
Ans: LPG is a better domestic fuel as it does not produce smoke and un-burnt carbon particles, which cause respiratory problems.
(c) Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Ans: Paper by itself catches fire easily because it has low ignition temperature but when wrapped around an aluminium pipe its temperature is lowered due to aluminium metal absorbing the heat supplied to paper. So it does not catch fire.
Q6. Make a labelled diagram of a candle flame.
Ans:
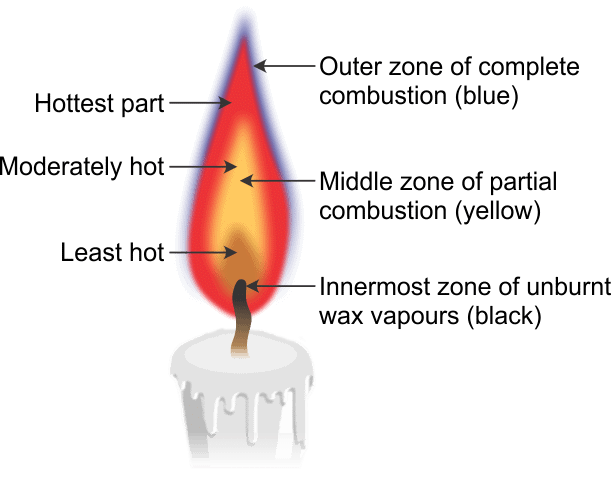
Q7. Name the unit in which the calorific value of a fuel is expressed.
Ans: The calorific value of a fuel is expressed in kilojoules per kilogram (kJ/kg).
Q8. Explain how CO2 is able to control fires.
Ans: Being heavier than oxygen, CO2 covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off, the fire is controlled.
Q9. It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Ans: Green leaves have a lot of moisture in them. This moisture does not allow them to catch fire easily. However, dry leaves have no moisture in them. Therefore, they catch fire easily.
Q10. Which zone of a flame does a goldsmith use for melting gold and silver and why?
Ans: A goldsmith uses the outer part of the candle flame for melting gold and silver because in this zone the temperature is the highest which helps to melt these metals easily.
Q11. In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Ans: Total mass of fuel = 4.5 kg
Heat produced by burning the given mass of fuel = 180,000 kJ.
We know that calorific value of fuel:
Hence, the calorific value of the given fuel = 40,000 kJ/kg.
Q12. Can the process of rusting be called combustion? Discuss.
 Rusted Iron Object
Rusted Iron Object
Ans:
Rusting is not truly combustion. Combustion is a fast reaction with oxygen that produces heat and often light, like burning wood. Rusting is a slow oxidation of iron with oxygen and water, releasing heat but no flames. Though both are exothermic, rusting lacks the speed and flame of combustion, making it a corrosion process instead.
Q13. Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time?
Ans: The water in the Ramesh's beaker will heat up in a shorter time. This is because the outermost zone of a flame is the hottest zone, while the yellow zone (in which Abida had kept the beaker) is less hot.
|
136 videos|530 docs|57 tests
|
FAQs on NCERT Solutions for Class 8 Science Chapter 4 - Combustion and Flame
| 1. What is combustion? |  |
| 2. What are the different types of combustion? |  |
| 3. What is a flame? |  |
| 4. What are the different methods of extinguishing a fire? |  |
| 5. What are the dangers of incomplete combustion? |  |

















