Antisymmetry Principle - Atomic Structure, Physical Chemistry, CSIR-NET - Government Jobs PDF Download
The Nature of Many-Electron Wavefunctions
Let us consider the nature of the electronic wavefunctions 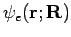 . Since the electronic wavefunction depends only parametrically on R, we will suppress R in our notation from now on. What do we require of
. Since the electronic wavefunction depends only parametrically on R, we will suppress R in our notation from now on. What do we require of 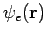 ? Recall that
? Recall that 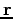 represents the set of all electronic coordinates, i.e.,
represents the set of all electronic coordinates, i.e., 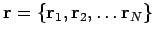 . So far we have left out one important item--we need to include the spin of each electron. We can define a new variable
. So far we have left out one important item--we need to include the spin of each electron. We can define a new variable 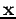 which represents the set of all four coordinates associated with an electron: three spatial coordinates
which represents the set of all four coordinates associated with an electron: three spatial coordinates 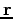 , and one spin coordinate
, and one spin coordinate 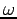 , i.e.,
, i.e., 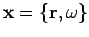 .
.
Thus we write the electronic wavefunction as 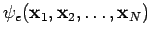 . Why have we been able to avoid including spin until now? Because the non-relativistic Hamiltonian does not include spin. Nevertheless, spin must be included so that the electronic wavefunction can satisfy a very important requirement, which is the antisymmetry principle (see Postulate 6 in Section 4). This principle states that for a system of fermions, the wavefunction must be antisymmetric with respect to the interchange of all (space and spin) coordinates of one fermion with those of another. That is,
. Why have we been able to avoid including spin until now? Because the non-relativistic Hamiltonian does not include spin. Nevertheless, spin must be included so that the electronic wavefunction can satisfy a very important requirement, which is the antisymmetry principle (see Postulate 6 in Section 4). This principle states that for a system of fermions, the wavefunction must be antisymmetric with respect to the interchange of all (space and spin) coordinates of one fermion with those of another. That is,
(184)
The Pauli exclusion principle is a direct consequence of the antisymmetry principle.
A very important step in simplifying 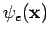 is to expand it in terms of a set of one-electron functions, or ``orbitals.'' This makes the electronic Schrödinger equation considerably easier to deal with. A spin orbital is a function of the space and spin coordinates of a single electron, while a spatial orbital is a function of a single electron's spatial coordinates only. We can write a spin orbital as a product of a spatial orbital one of the two spin functions
is to expand it in terms of a set of one-electron functions, or ``orbitals.'' This makes the electronic Schrödinger equation considerably easier to deal with. A spin orbital is a function of the space and spin coordinates of a single electron, while a spatial orbital is a function of a single electron's spatial coordinates only. We can write a spin orbital as a product of a spatial orbital one of the two spin functions
(185)
(186)
Note that for a given spatial orbital 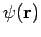 , we can form two spin orbitals, one with
, we can form two spin orbitals, one with 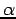 spin, and one with β spin. The spatial orbital will be doubly occupied. It is possible (although sometimes frowned upon) to use one set of spatial orbitals for spin orbitals with
spin, and one with β spin. The spatial orbital will be doubly occupied. It is possible (although sometimes frowned upon) to use one set of spatial orbitals for spin orbitals with 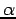 spin and another set for spin orbitals with β spin.
spin and another set for spin orbitals with β spin.
Where do we get the one-particle spatial orbitals 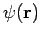 ? That is beyond the scope of the current section, but we briefly itemize some of the more common possibilities:
? That is beyond the scope of the current section, but we briefly itemize some of the more common possibilities:
- Orbitals centered on each atom (atomic orbitals).
- Orbitals centered on each atom but also symmetry-adapted to have the correct point-group symmetry species (symmetry orbitals).
- Molecular orbitals obtained from a Hartree-Fock procedure.
We now explain how an 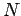 -electron function
-electron function 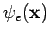 can be constructed from spin orbitals, following the arguments of Szabo and Ostlund (p. 60). Assume we have a complete set of functions of a single variable
can be constructed from spin orbitals, following the arguments of Szabo and Ostlund (p. 60). Assume we have a complete set of functions of a single variable 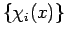 . Then any function of a single variable can be expanded exactly as
. Then any function of a single variable can be expanded exactly as
(187)
How can we expand a function of two variables, e.g. 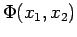 ?
?
If we hold x2 fixed, then
(188)
Now note that each expansion coefficient 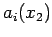 is a function of a single variable, which can be expanded as
is a function of a single variable, which can be expanded as
(189)
Substituting this expression into the one for 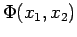 , we now have
, we now have
(190)
a process which can obviously be extended for 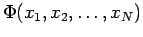 .
.
We can extend these arguments to the case of having a complete set of functions of the variable 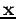 (recall
(recall 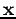 represents x, y, and z and also
represents x, y, and z and also 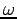 ). In that case, we obtain an analogous result,
). In that case, we obtain an analogous result,
(191)
Now we must make sure that the antisymmetry principle is obeyed. For the two-particle case, the requirement
(192)
implies that 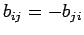 and
and 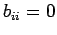 , or
, or
(193)
where we have used the symbol 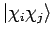 to represent a Slater determinant, which in the genreral case is written
to represent a Slater determinant, which in the genreral case is written
(194)
We can extend the reasoning applied here to the case of 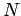 electrons; any
electrons; any 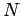 -electron wavefunction can be expressed exactly as a linear combination of all possible
-electron wavefunction can be expressed exactly as a linear combination of all possible 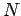 -electron Slater determinants formed from a complete set of spin orbitals
-electron Slater determinants formed from a complete set of spin orbitals 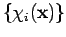 .
.
FAQs on Antisymmetry Principle - Atomic Structure, Physical Chemistry, CSIR-NET - Government Jobs
| 1. What is the antisymmetry principle in atomic structure? |  |
| 2. How does the antisymmetry principle affect the electron configuration of atoms? |  |
| 3. Why is the antisymmetry principle important in physical chemistry? |  |
| 4. How does the antisymmetry principle relate to the CSIR-NET exam? |  |
| 5. What are some examples of the antisymmetry principle in action? |  |


















