Theory & Procedure, Qualitative Analysis of Cations | Additional Study Material for NEET PDF Download
Our Objective
Our objective is to determine the cation present in a given salt.
The Theory
What is Qualitative Analysis?
Qualitative analysis is a method of Analytical chemistry that deals with the determination of elemental composition of inorganic salts. It is mainly concerned with the detection of ions in an aqueous solution of the salt.
The common procedure for testing any unknown sample is to make its solution and test this solution with various reagents for the ions present in it. Testing with various reagents gives characteristic reaction of certain ions, which may be a colour change, a solid formation or any other visible changes. There are separate procedures for detecting cations and anions, called the Cation Analysis and Anion Analysis.
Let us discuss about the Qualitative Analysis of Cations.
Qualitative Analysis of Cations
Preliminary Tests
Some preliminary tests needs to be done before doing the analysis of cations.
(a) Physical Appearance: Colour and Smell
The physical examination of the unknown salt involves the study of colour, smell and density. The test is not much reliable, but certainly helpful in identifying some coloured cations. Characteristic smell helps to identify some ions like ammonium ion.
(b) Charcoal Cavity Test
This test is based on the fact that metallic carbonates when heated in a charcoal cavity decomposes to give corresponding oxides. The oxides appear as coloured incrustation or residue in the cavity. In certain cases, the oxides formed partially undergo reduction to the metallic state producing metallic beads or scales.
Examples:
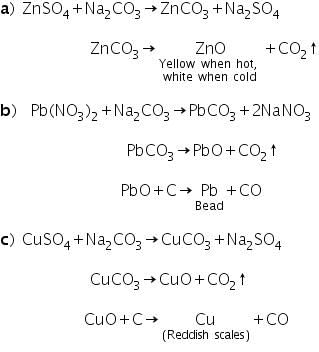
(c) Cobalt Nitrate Test (Ash test)
This test is applied to those salts that leave white residue in the charcoal cavity test. This test is based on the fact that metallic carbonates when heated in a charcoal cavity decomposes to give corresponding oxides.
The test is based on the fact that cobalt nitrate decomposes on heating to give cobalt oxide, CoO. This combines with the metallic-oxides present as white residue in the charcoal cavity forming coloured compounds.
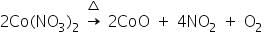
For example, when a magnesium salt undergoes charcoal cavity test, a while residue of MgO is left behind. This on treatment with cobalt nitrate and on subsequent heating forms a double salt of the formula MgO.CoO, which is pink in colour.
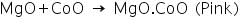
Other examples are:
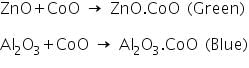
In addition to metallic oxides, phosphates and borates also react with cobalt oxide to form Co3(PO4)2 and Co3(BO3)2 that are blue in colour.
(d) Flame Test
Certain salts on reacting with conc. HCl forms their chloride, that are volatile in non-luminous flame. Their vapours impart characteristic colour to the flame. This colour can give reliable information of the presence of certain cations . For proceeding to this test, the paste of the mixture with conc.HCl is introduced into the flame using a platinum wire.
(e) Borax Bead Test
Borax, Na2B4O7.10H2O, on heating gets fused and loses water of crystallisation. It swells up into a fluffy white porous mass which then melts into a colourless liquid that later forms a clear transparent glassy bead consisting of boric anhydride and sodium metaborate.
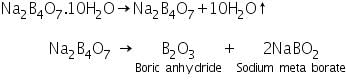
Boric anhydride is non-volatile. When it is made to react with coloured metallic salt, a characteristic coloured bead of metal metaborate is formed. In those cases where different coloured beads are obtained in oxidising and reducing flames, metaborates in various oxidation states of metals are formed. For example, in oxidising flame, copper forms blue copper metaborate.
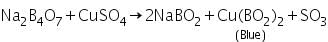
In reducing flame, cupric metaborate is reduced to metallic copper, which is red and opaque.
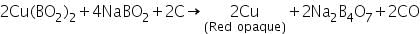
Identification of Cations (Basic Radicals)
Analysis of Group Zero
This group includes ammonium ion (NH4+).
Chemical reactions involved in Group Zero analysis
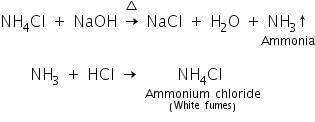
(a) Sodium hydroxide test
When ammonium salt is heated with conc. NaOH, ammonia gas is evolved which gives white fumes with dil.HCl due to the formation of NH4Cl.
(b) Nessler's reagent test
Tha ammonia gas formed by the reaction of ammonium ions with NaOH reacts with Nessler's reagent to form a brown precipitate of H2N.HgO.HgI.

Analysis of Group I
This group includes Pb2+, Ag+, Hg22+. Here we shall study only Pb2+. Group reagent for this group is dil. HCl.
Chemical reactions involved in Group I analysis
The addition of HCl to the solution will precipitate Pb2+ as lead chloride which is soluble in hot water. On cooling, the precipitate settle down as PbCl2 which is less soluble in cold water.
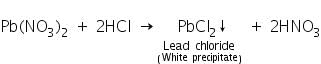
Confirmatory tests of Lead (II) ion (Pb2+)
(a) Potassium iodide test
Lead chloride (formed by the reaction of lead salts with dil. HCl) solution in hot water reacts with potassium iodide solution to form yellow precipitate of lead iodide.

(b) Potassium chromate test
Lead chloride (formed by the reaction of lead salts with dil. HCl) solution in hot water reacts with solution of potassium chromate to form yellow precipitate of lead chromate.
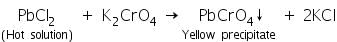
Analysis of Group II
This group includes Pb2+ and Cu2+ in IIA Group and As3+ in IIB Group.
Chemical reactions involved in Group II Analysis:
Passing of H2S gas through the acidified original solution will precipitate the radicals Pb2+,Cu2+,As3+ as their sulphides.
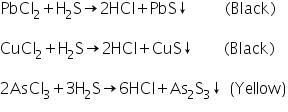
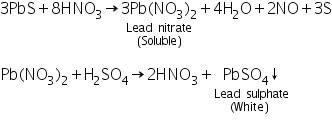
Confirmatory tests for Lead (II) ion (Pb2+)
Black precipitate of PbS formed in the group analysis dissolves in 50% nitric acid due to the formation of soluble lead nitrate. On adding sulphuric acid to the soluble lead nitrate, lead sulphate precipitates.
(a) Potassium iodide test
The soluble lead nitrate (formed by the reaction between PbS and 50% Conc. HNO3) reacts with potassium iodide to form yellow precipitate of lead iodide.
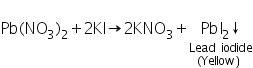
(b) Potassium chromate test
The soluble lead nitrate (formed by the reaction between PbS and 50% Conc.HNO3) reacts with potassium chromate to form yellow precipitate of lead chromate.
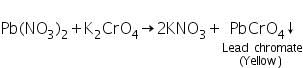
Confirmatory tests for Copper (II) ion (Cu2+)
Black precipitate of CuS formed in the group analysis dissolves in 50% nitric acid and a blue solution is obtained on addition excess of NH4OH.
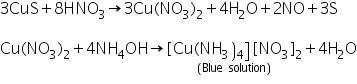
(a) Potassium ferrocyanide test
The above blue solution gives a chocolate brown precipitate with potassium ferrocyanide solurion.

(b) Potassium iodide test
In this test, the white precipitate is due to the formation of cuprous iodide and the brown colour of the solution is due to liberation of iodine.
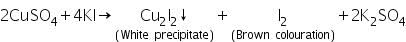
Confirmatory tests of Arsenic (III) ion (As3+)
The yelllow residue of As2S3 formed in the group analysis is dissolved in conc.HNO3 forming arsenic acid.
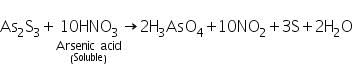
(a) Ammonium molybdate test
Arsenic acid ( formed by dissolving As2S3 in conc.HNO3) reacts with ammonium molybdate to form yellow precipitate of ammonium arseno molybdate.
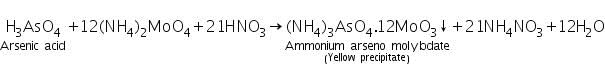
(b) Magnesia mixture test
Arsenic acid ( formed by dissolving As2S3 in conc.HNO3) reacts with magnesia mixture to form a white precipitate of Mg(NH4)2AsO4.
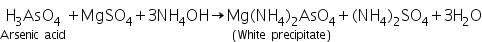
Analysis of Group - III
The cations present in this group are Fe2+, Fe3+, Cr3+ and Al3+. We will look at only Fe2+/ Fe3+ and Al3+.
Chemical Reactions Involved Group III Analysis
The cations in this group are precipitated as hydroxides by adding ammonium hydroxide in presence of ammonium chloride. Thus, group reagent for this group is NH4OH in the presence of NH4Cl.
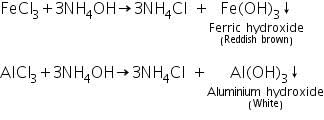
Confirmatory tests of Ferric ion (Fe3+)
The reddish brown precipitate of ferric hydroxide is dissolved in HCl due to the formation of soluble ferric chloride.
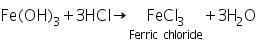
(a) Potassium ferrocyanide test
Ferric chloride (formed by dissolving ferric hydroxide in HCl) reacts with potassium ferrocyanide to form prussian blue coloured Ferric ferrocyanide.
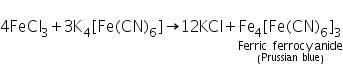
(b) Potassium sulphocyanide test
Ferric chloride (formed by dissolving ferric hydroxide in HCl) reacts with potassium sulphocyanide to form blood red coloured Ferric sulphocyanide.
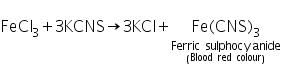
Confirmatory tests of Aluminium (III) ion ( Al3+)
(a) Lake test
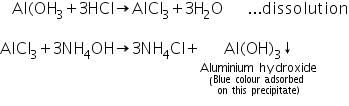
Aluminium hydroxide formed in the group analysis dissolves in dil. HCl to form soluble aluminium chloride. The aluminium chloride thus formed reacts with ammonium hydroxide to reform aluminium hydroxide. Blue colour of litmus solution is adosrbed on this precipitate.
(b) Charcoal cavity/Cobalt nitrate test
In this test, aluminium oxide produced in the charcoal cavity test reacts with CoO in cobalt nitrate test to produces a blue mass due to the formation of Al2O3.CoO.
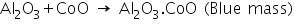
Analysis of Group - IV
The radicals present in this group are CO2+, Ni2+,Mn2+ and Zn2+. These are precipitated as sulphide by passing H2S gas through the ammonical solution of the salt. The group reagent for this group is H2S gas in the presence of NH4Cl and NH4OH.
Chemical Reaction involved in Group IV Analysis
Passing of H2S gas through the group III solution will precipitate the radicals Co2+, Ni2+, Mn2+ and Zn2+ as their sulphides. Formation of black ppt. (CoS or NiS) indicates cobalt or nickel. Formation of buff-coloured ppt. (MnS) indicates manganese and dirty white ppt. (ZnS) indicates zinc.

Confirmatory tests of Cobalt (II) ion (Co2+)
(a) Potassium nitrite test
Cobalt ion reacts with potassium nitrite in presence of acetic acid to form yellow precipitate of Potassium cobalti nitrite.
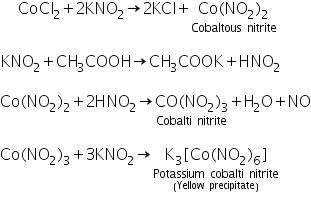
(b) Ammonium thiocyanate ether test
On addition of ether and a crystal of ammonium thiocyanate, a blue colour is obtained in the ethereal layer due to the formation of ammonium cobalti thiocyanate.
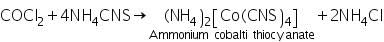
(c) Borax bead test
In this test Co2+ ion produces blue beads in both oxidising and reducing flames.
Confirmatory tests of Nickel (II) ion (Ni2+)
(a) Dimethyl glyoxime test
Ni2+ ions react with dimethyl glyoxime to form bright rose-red coloured Nickel-Dimethyl glyoxime complex, Ni(dmgH)2.
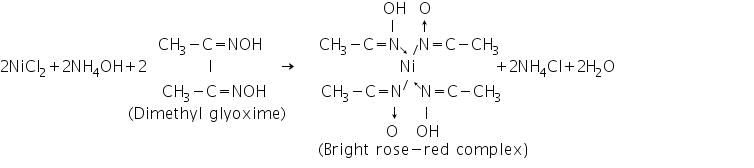
(b) Sodium hydroxide-bromine water test
Ni2+ ions react with excess of NaOH and bromine water to form a black precipitate of Nickelic hydroxide.
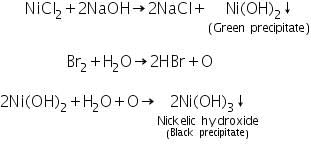
(c) Borax bead test
Ni2+ ions produce brown bead in oxidising and grey bead in reducing flames.
Confirmatory tests of Manganese (II) ion (Mn2+)
Manganese sulphide formed in the group analysis dissolves in dil.HCl forming manganese chloride, and H2S is boiled off.
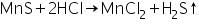
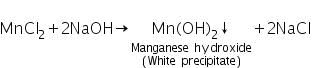
(a) Sodium hydroxide - bromine water test
The manganese chloride (formed by dissolving MnS in HCl) reacts with excess of NaOH to form white precipitate of manganese hydroxide.
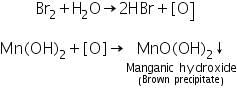
The white precipitate of manganese hydroxide turns brown on addition of bromine water due to its oxidation to brown manganic hydroxide MnO(OH)2.
(b) Lead peroxide test
Manganese sulphide formed in the group analysis reacts with Conc. HNO3 and lead peroxide to form a pink coloured solution, due to the formation of HMnO4.
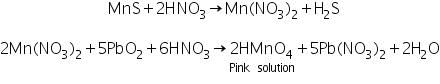
(c) Borax bead test
Mn2+ ions produce pinkish beads in oxidising and colourless beads in reducing flames.
Confirmatory tests of Zinc (II) ion (Zn2+)
The white precipitate of ZnS formed in the group analysis dissolves in dil. HCl forming zinc chloride, and H2S is boiled off.
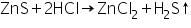
(a) Sodium hydroxide test
Zinc chloride (formed by dissolving ZnS in dil. HCl) reacts with sodium hydroxide to form white precipitate of zinc hydroxide.

(b) Potassium ferrocyanide test
Zinc chloride (formed by dissolving ZnS in dil. HCl) reacts with potassium ferrocyanide to form white or bluish white precipitate of zinc ferrocyanide.
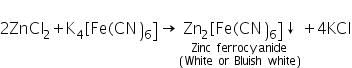
(c) Charcoal cavity/Cobalt nitrate test
In this test, zinc oxide produced in the charcoal cavity test reacts with CoO in cobalt nitrate test to produces a greenish residue due to the formation of ZnO.CoO.
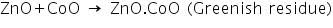
Analysis of Group - V
Group V consist of three radicals: Ba2+, Sr2+ and Ca2+. These cations are precipitated as their carbonates.Group reagent for this group is (NH4)2CO3 in the presence of NH4Cl and NH4OH.
Chemical Reaction involved in Group V Analysis
When (NH4)2CO3 is added to salt solution containing NH4Cl and NH4OH, the carbonates of Ba2+, Sr2+ and Ca2+ are precipitated.
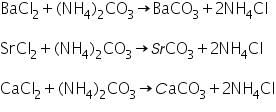
Confirmation of Barium (II) ion (Ba2+)
The white precipitate of barium carbonate formed in the group analysis dissolves in hot dil. acetic acid due to the formation of soluble barium acetate.
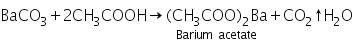
(a) Potassium chromate test
Barium acetate (formed by dissolving barium carbonate in dil. acetic acid) reacts with potassium chromate to form yellow precipitate of barium chromate.
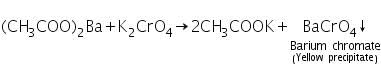
(b) Flame test
Barium imparts a grassy green colour to the flame.
Confirmation of Strontium (II) ion (Sr2+)
The white precipitate of strontium carbonate formed in the group analysis dissolves in hot dil. acetic acid due to the formation of soluble strontium acetate.
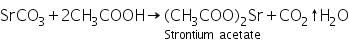
(a) Ammonium sulphate test
Strontium acetate ( formed by dissolving Strontium carbonate in dil. acetic acid ) reacts with ammonium sulphate to form a white precipitate o strontium sulphate.
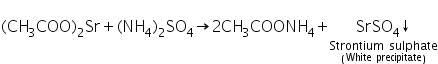
(b) Flame test
Strontium imparts a crimson red colour to the flame.
Confirmation of Calcium (II) ion (Ca2+)
The white precipitate of calcium carbonate formed in the group analysis dissolves in hot dil. acetic acid due to the formation of soluble calcium acetate.
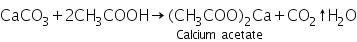
(a) Ammonium oxalate test
Calcium acetate (formed by dissolving calcium carbonate in dil. acetic acid) reacts with ammonium oxalate to form a white precipitate o calcium oxalate.
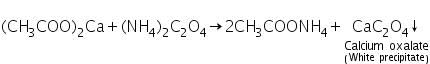
(b) Flame test
Calcium imparts brick red colour to the flame.
Analysis of Group - VI
Chemical Reaction involved in Confirmation of Mg2+
(a) Ammonium Phosphate test
Mg2+ ions react with ammonium phosphate in presence of NH4Cl and NH4OH to form white precipitate of magnesium ammonium phosphate.
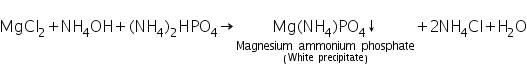
(b) Charcoal cavity/Cobalt nitrate test
In this test, magnesium oxide produced in the charcoal cavity test reacts with CoO in cobalt nitrate test to produces a pink mass due to the formation of MgO.CoO.
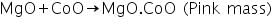
Learning Outcomes
- Students understand different types of cations.
- Students understand various tests to determine the cation present in a given salt.
- Students understand the chemical reactions and their balanced equations that takes place during each test.
- Students acquire the skill to perform the experiment in the real lab once they understand different steps in the procedure.
Real Lab Procedure
Preliminary Tests
Physical Examination
Experiment | Observation | Inference |
Colour:Note down the colour of the given salt. | Blue or Bluish green | May be Cu2+ or Ni2+ |
Greenish | May be Ni2+ | |
Light Green | May be Fe2+ | |
Dark brown | May be Fe3+ | |
Pink | May be Co2+ | |
Light pink,flesh colour or earthy colour | May be Mn2+ | |
White | May be Cu2+, Ni2+, Fe2+, Fe3+ and Co2+ etc are absent. | |
Smell: Take a pinch of the salt between your fingers and rub with a drop of water. | Ammoniacal smell | May be NH4+ |
Density | Heavy | May be the salt of Pb2+ or Ba2+ |
Deliquescence | Salt absorbs moisture and becomes paste like. | If coloured, may be Cu(NO3)2 ot FeCl3. |
If colourless, Maly be Zn(NO3)2, chlorides of Zn2+, Mg2+ etc. |
Charcoal Cavity Test
Experiment | Observation | Inference | ||
First make a small cavity on a charcoal box using a borer. Mix a small quantity of the salt with double its quantity of sodium carbonate in a watch glass. Place the mixture in the cavity made on the block of charcoal. Moisten the mixture with a drop of water. Direct the reducing flame of the Bunsen burner on the cavity by means of a mouth blowpipe. Heat strongly for sometime and record the observations. | Residue | Metallic bead | ||
Hot | Cold | |||
Yellow | White | None | Zn2+ | |
Brown | Yellow | Grey bead which marks the paper | Pb2+ | |
None | None | Red beads or scales | Cu2+ | |
White residue which glows | None | None | Ba2+, Ca2+, Mg2+ | |
Black | None | None | Nothing definite - generally coloured salt | |
Note: To obtain a reducing flame, with the help of a mouth blow pipe make the Bunsen burner flame luminous by closing the air holes of the burner. Keep the nozzle of the blow pipe just outside the flame and blow gently on to the cavity.
Cobalt Nitrate Test (Ash Test)
Experiment | Observation (colour of the residue) | Inference |
Put one or two drops of cobalt nitrate solution on the white residue left after charcoal cavity test. Heat for one or two minutes using a blow pipe in oxidising flame. Observe the colour of the residue and draw the inference. | Green | Zn2+ |
Pink | Mg2+ | |
Blue | Al3+ | |
Black | It is due to the formation of CoO. No definite indication. |
Note:
- Perform the cobalt nitrate tset only if the residue in the charcoal cavity test is white.
- Do not put more than 2 drops of cobalt nitrate on the white residue during cobalt nitrate test. Excess cobalt nitrate may decompose to give cobalt oxide which is black in colour.
- Use dilute solution of cobalt nitrate during cobalt nitrate test.
Flame test
Experiment | Observation(colour of the flame) | Inference |
Clean the platinum wire by dipping it in conc.HCl taken in a watch glass and then heat it strongly in the flame. This process is repeated till the wire imparts no colour to the flame. Now prepare a paste of the mixture with conc.HCl, in a clean watch glass. Place a small amount of this paste on the platinum wire loop and introduce it into the flame. Note the colour imparted to the flame. | Brick - red (not persistent) | Ca2+ |
Crimson - red (persistent) | Sr2+ | |
Grassy - green (Persistent) | Ba2+ | |
Bright bluish - gree | Cu2+ | |
Green flashes | Zn2+ or Mn2+ | |
Dull bluish - white | Pb2+ |
Before carrying out the confirmatory tests for analysis of cations, the salt has to be dissolved in some suitable solvent to prepare its solution.
Preparation of Solution for Confirmatory Tests of Cations
The very first essential step is to prepare a clear and transparent solution of the salt under investigation. For this purpose, the following solvents are tried one after another in a systematic order.
- Distilled water (cold or hot)
- Dilute HCl (cold or hot)
- Conc. HCl (cold or hot)
In case the salt does not dissolve in a particular solvent even on heating, try the next solvent.
Procedure
Take a small quantity of the given salt in a test tube. Add some suitable solvent to it and shake well. If it does not dissolve, heat the contents gently for sometime. If it does not dissolve even after heating for sometime, take the fresh quantity of the salt again and treat it in a similar manner with next solvent. The clear solution thus obtained is labelled as Original Solution (O.S).
Analysis of Group-Zero (NH4+)
Experiment | Observation | Inference |
To a small amount of solid salt taken in a test tube, add some concentrated solution of sodium hydroxide and heat the contents. | Characteristic ammoniacal smell. | The gas evolved is NH3. Presence of Group Zero (NH4+). |
Experiment | Observation | Inference |
To a small amount of solid salt taken in a test tube, add some concentrated solution of sodium hydroxide and heat the contents. Bring a glass rod dipped in dil. HCl near the mouth of the test tube. | A gas with ammoniacal smell is evolved. White fumes is produced. | The gas evolved is ammonia which gives white fumes with HCl due to the formation of NH4Cl. Presence of NH4+ is confirmed. |
Nessler’s Reagent test: When the gas evolved in the above test is passed through Nessler's reagent taken in a test tube. | Brown precipitate is formed | The brown precipitate is due to the formation of H2N.HgO.HgI. Presence of NH4+ is confirmed. |
Analysis of Group I
Experiment | Observation | Inference |
To a small amount of salt solution taken in a test tube, add dil. hydrochloric acid. Centrifuge and wash the precipitate. | White precipitate is formed | The white precipitate may due to the formation of PbCl2. Presence of group I (Pb2+). |
Confirmation of Pb2+
Experiment | Observation | Inference |
Boil the white precipitate with 5-10 m of water - Precipitate dissolves - PbCl2 is soluble in hot water - Divide the solution into three parts. | ||
1.Cool one part of the solution. | White crystalline precipitate is formed. | On cooling, precipitate settle down as PbCl2. Presence of Pb2+ ion is confirmed. |
2. Potassium iodide test: To the second part of the solution, add potassium iodide solution. | Yellow precipitate is formed. | Yellow precipitate is due to the formation of PbI2. Presence of Pb2+ ion is confirmed. |
3. Potassium chromate test: To the third part of the solution add potassium chromate solution. | Yellow precipitate is formed. | Yellow precipitate is due to the formation of PbCrO4. Presence of Pb2+ ion is confirmed. |
Note:
- If the original solution is prepared in cold dilute hydrochloric acid, first group is absent.
- If the original solution is prepared in conc. hydrochloric acid, simply add water. White ppt. shows the presence of first group.
Analysis of Group II (Copper Group)
Experiment | Observation | Inference |
Take about 2 ml of the original solution in a test tube. Add some dil. HCl and warm the contents. Through this solution pass H2S gas from the Kipp's apparatus by pressing the nozzle. | Formation of the black precipitate. | The black precipitate may be due to the formation of PbS or CuS. Presence of Group II (Pb2+ or Cu2+). |
Formation of the yellow precipitate. | The yellow precipitate may be due to the formation of As2S3.Presence of Group II (As3+). | |
Centrifuge and separate the precipitate. | ||
Black precipitate (Pb2+ or Cu2+) Heat the black precipitate with minimum quantity (1-2 ml) of 50% HNO3 in a tests tube - Precipitate dissolves. Inference:Black precipitate dissolves in 50% HNO3 either due to the formation of Pb(NO3)2 or due to the formation of Cu(NO3)2. To one part of the above solution, add dil. H2SO4 and alcohol. | Yellow precipitate As3+ | |
White precipitate – Inference: On adding H2SO4, white precipitate of lead sulphate, PbSO4 is formed. Dissolve the precipitate in hot ammonium acetate solution and divide the solution into two parts: | No white precipitate. To rest of the solution add NH4OH in excess - Blue coloured solution (Cu2+) Inference:The blue coloured solution is due to the formation of [Cu(NH3)4][NO3]2 by the reaction between Cu(NO3)2 and NH4OH. | Add some Conc. HNO3 into the yellow precipitate in a test tube –Precipitate dissolves. Inference:The yellow residue of As2S3 is dissolved in Conc. HNO3 forming arsenic acid, H3AsO4. Divide the solution into two parts. |
Confirmation | Confirmation | Confirmation |
1. Potassium iodide test: To one part of the solution in a test tube add potassium iodide solution - Yellow precipitate is formed -The ppt dissolves in boiling water and on cooling recrystalises. Inference: Yellow precipitate is due to the formation of Lead iodide, PbI2. Presence of Pb2+is confirmed. | 1. Potassium ferrocyanide test: To one part of the blue solution in a test tube add few drops of acetic acid and potassium ferrocyanide solution - Chocolate brown precipitate is formed. Inference: The chocolate brown precipitate is due to the formation of Cu2[Fe(CN)6]. Presence of Cu2+ is confirmed. | 1. Ammonium molybdate test: To a part of the solution in a test tube, add ammonium molybdate solution and heat - Yellow precipitateis formed. Inference:Yellow precipitate is due to the formation of ammonium arseno molybdate {(NH4)3AsO4.12MoO3}. Presence of As3+ is confirmed. |
2. Potassium chromate test: To another part of the solution, add potassium chromate solution.–Yellow precipitate is formed –Add some NaOH solution to this precipitate –Precipitate dissolves. Inference:Yellow precipitate is due to the formation of lead chromate. Presence of Pb2+ is confirmed. | 2. Potassium iodide test: To another part of the blue solution add acetic acid and potassium iodide solution -White precipitate is formed in brown coloured solution. Inference: White precipitate formed is Cu2I2. Brown colour of the solution is due to the formation of iodine. Presence of Cu2+ is confirmed. | 2. Magnesia mixture test: To the second part of the solution, add NH4OH solution to make it alkaline and add magnesia mixture - White precipitate is formed. Inference:The white precipitate is due to the formation of Mg(NH4)2AsO4. Presence of As3+ is confirmed. |
In case, first and second groups are absent proceed for group III with the original solution.
Experiment | Observation | Inference |
Take about 5 ml of salt solution in a test tube and add 4-5 drops of conc. HNO3. Boil the solution for some time. Add to it about 2 g of solid NH4Cl and boil again. Cool the solution by placing the test tube in a beaker full of water. Add excess of ammonium hydroxide to it and shake. | Reddish brown precipitate. | Reddish brown precipitate may be due to the formation of ferric hydroxide, Fe(OH)3. Presence of Group III cation (Fe3+). |
White precipitate. | White precipitate may be due to the formation of aluminium hydroxide, Al(OH)3. Presence of Group III cation (Al3+) | |
Centrifuge and separate out the precipitate. | ||
Reddish Brown precipitate (Fe3+) | White precipitate (Al3+) |
Dissolve the reddish brown ppt. in dilute HCl, and divide the solution into two parts. | |
Confirmation | Confirmation |
1. Pottassium ferrocynaide test: To one part of the above solution in a test tube add potassium ferrocyanide solution - Prussian blue colouration. Inference: Prussian blue colour is due to the formation of ferric ferrocyanide, Fe4[Fe(CN)6]3. Presence of Fe3+ is confirmed. | 1. Lake test: Disolve the white ppt. in dilute hydrochloric acid. Add to it two drops of blue litmus solution. To this, add NH4OH dropwise till blue colour develops - Blue precipitate floating in colourless solution. Inference: The precipitate formed is aluminium hydroxide. Blue colour absorbs on this precipitate. Presence of Al3+ is confirmed. |
2. Potassium sulphocyanide test: To the second part of the solution, add a little potassium sulphocyanide solution - Blood red colouration. Inference:Blood red colouration is due to the formation of Ferric sulphocyanide, Fe(CNS)3. Presence of Fe3+ is confirmed. | 2. Cobalt nitrate/Charcoal cavity test: Take a charcoal box with a small cavity in it. Take a small amount of salt in a watch glass. Mix it with solid sodium carbonate whose quantity is 2 times that of the salt. Put this mixture in the cavity. Add a drop of water to the mixture. Then direct the reducing flame of the Bunsen burner on the cavity using the blowpipe. Heat strongly for sometime - A white residue is formed. Put one or two drops of cobalt nitrate solution on the white residue left after the cavity in the charcoal cavity test. Direct oxidizing flame into the mixture using blow pipe and observe the colour of the residue - Blue mass is formed. Inference: The blue mass is due to the formation of Al2O3.CoO. Presence of Al3+ is confirmed. |
If there is no ppt. in the third group, then use the same ammonical solution for the fourth group.
Experiment | Observation | Inference |
Pass H2S gas through the Ammonical solution. {Ammoniacal solution: - Take about 5 ml of salt solution in a test tube and add 4-5 drops of conc. HNO3. Boil the solution for some time. Add to it about 2 g of solid NH4Cl and boil again. Cool the solution by placing the test tube in a beaker full of water. Add excess of ammonium hydroxide to it and shake}. | Black precipitate | Black precipitate may be due to the formation of CoS or NiS. Presence of Group IV cation. |
Buff (flesh) coloured precipitate | Buff coloured precipitate may be due to the formation of MnS. Presence of Group cation. | |
Dull white precipitate | Dull white precipitate may be due to the formation of ZnS. Presence of Group IV cation. |
Black precipitate (Co2+ or Ni) Observe the colour of the original salt. If the salt is purple or deep violet in colour, perform confirmatory tests for Co2+ and if it is greenish, perform confirmatory tests for Ni2+ with the original solution. | Buff (f2+lesh) coloured precipitate (Mn2+) | Dull white precipitate (Zn2+) To the dull white precipitate, add some dil. HCl and heat the contents – The precipitate dissolves with the evolution of H2S gas. Divide the solution into two parts. Inference: The white precipitate of ZnS dissolves in dil. HCl to form ZnCl2 with the evolution of H2S gas. | |
Confirmation of Co2+ | Confirmation of Ni2+ | Confirmation of Mn2+ | Confirmation of Zn2+ |
1. Potassium nitrite test: To one part of the original salt solution in a tests tube, add ammonium hydroxide to neutralize the solution. Add acetic acid and a crystal of potassium nitrite. Warm the test tube - Yellow precipitate is formed. Inference: The yellow precipitate is due to the formation of potassium cobalti nitrite, K3[Co(NO2)6]. Presence of Co2+ is confirmed. | 1. Dimethyl glyoxime test: To one part of the original salt solution taken in a test tube, add ammonium hydroxide solution and few drops of dimethyl glyoxime - Bright rose red precipitate is obtained. Inference: The bright red colour is due to the formation of Ni – dimethyl glyoxime complex; Ni(dmgH)2. Presence of Ni2+is confirmed. | 1. Sodium hydroxide-Br2 test: To one part of salt solution in a test tube, add NaOH solution - A white precipitate is formed - Add Bromine water to white precipitate - White precipitate turns black or brown. Inference:White precipitate is due to the formation of manganese hydroxide, Mn(OH)2.Mn(OH)2turns brown on adding Br2 water due to the oxidation of Mn(OH)2 to MnO(OH)2. Presence of Mn2+ is confirmed. | 1. Sodium hydroxide test: To one part of the above solution in a test tube, add sodium hydroxide (NaOH) solution dropwise - A white precipitate is formed - Add more NaOH to the white precipitate - The white ppt. dissolves. Inference:The white precipitate is due to the formation of zinc hydroxide, Zn(OH)2 which is soluble in excess NaOH due to the formation of Na2ZnO2. Presence of Zn2+ is confirmed. |
2. Ammonium thiocyanate ether test: To another part of the original salt solution in a test tube, add about 1 ml of ether. Add crystals of ammonium thiocyanate and shake the test tube well. Keep the solution undisturbed for some time –Blue colouration in the ethereal layer. Inference:Blue colour is due to the formation of ammonium cobalti thiocyanate, (NH4)2[Co(CNS)4]. Presence of Co2+ is confirmed. | 2. Sodium hydroxide - Br2test: To another part of the original salt solution in a test tube, add sodium hydroxide (in excess) – Green precipitate is formed - Add bromine water to the above ppt.. Boil the content - A black precipitate is formed. Inference:The green precipitate is due to the formation of Ni(OH)2.The black precipitate is due to the formation of nickelic hydroxide, Ni(OH)3. Presence of Ni2+ is confirmed. | 2. Lead peroxide test: To black ppt. obtained in above test (Sodium hydroxide-Br2 test), add conc. HNO3 and lead peroxide solution. Boil, cool and allow to settle - Pink coloured solution is formed. Inference:The pink colour is due to the formation of HMnO4. Presence of Mn2+ is confirmed. | 2. Potassium ferrocyanide test: To the second part above solution in a test tube, add potassium ferrocyanide solution - White or bluish white precipitate is formed. Inference: The white or bluish white precipitate is due to the formation of Zn2[Fe(CN)6]. Presence of Zn2+ is confirmed. |
3. Borax bead test:Take some borax using the loop of platimun wire and heat it on a burner, it swells and form transparent colourless glassy bead. Touch the hot bead in the platinum wire with the coloured salt and is heated again in both oxidising and reducing flames. Note colour of the beads in both flames.- A deep blue bead is obtained in oxidising and reducing flames. Inference:Presence of Co2+is confirmed. | 3. Borax bead test: Take some borax using the loop of platimun wire and heat it on a burner, it swells and form transparent colourless glassy bead. Touch the hot bead in the platinum wire with the coloured salt and is heated again in both oxidising and reducing flames. Note colour of the beads in both flames.- A brown bead in oxidizing and grey bead in reducing flame is obtained. Inference:Presence of Ni2+ is confirmed. | 3. Borax bead test: Take some borax using the loop of platimun wire and heat it on a burner, it swells and form transparent colourless glassy bead. Touch the hot bead in the platinum wire with the coloured salt and is heated again in both oxidising and reducing flames. Note colour of the beads in both flames-A pinkish on oxidising and colourless bead on reducing flames. Inference: Presence of Mn2+ is confirmed. | 2. Cobalt nitrate/Charcoal cavity test: Take a charcoal box with a small cavity in it. Take a small amount of salt in a wach glass. Add solid sodium carbonate whose quantity is 2 time that of the salt into the cavity. Put this mixture in the cavity. Add a drop of water to the mixture. Then direct the reducing flame of Bunsen burner on the cavity by means of blowpipe. Heat strongly for sometime - A white residue is formed. Put one or two drops of cobalt nitrate solution on the white residue left after the cavity in the charcoal cavity test. Direct oxidizing flame into the mixture using blow pipe and observe the colour of the residue - Green mass is formed. Inference:The blue mass is due to the formation of ZnO.CoO. Presence of Zn2+ is confirmed. |
If the fourth group is absent, then proceed for radicals of group V.
Experiment | Observation | Inference |
To the original salt solution, add 2-3 grams of solid NH4Cl, boil and cool the contents and add NH4OH till the solution smells ammonia. Then add ammonium carbonate solution. Centrifuge the precipitate and wash with water. Add hot dilute acetic acid into the precipitate. Divide the solution into three parts. | White precipitate is formed and which is dissolved in hot dil. acetic acid.. | Th white precipitate may due to the formation of carbonates of Ba2+, Sr2+ or Ca2+. These insoluble carbonate dissolves in acetic acid due to the formation of soluble acetates of Ba2+, Sr2+ or Ca2+. Presence of Group V cation. |
Confirmaton of Ba2+ | Confirmation of Sr2+ | Confirmation of Ca2+ |
1. Potassium chromate test: To one part of the above solution in a test tube, add a few drops of potassium chromate solution - Yellow precipitate is formed. Inference:The yellow precipitate is due to the formation of barium chromate, BaCrO4. Presence of Ba2+ is confirmed. | 1. Ammonium sulphate test: To second part of the above solution in a test tube, add 1 ml of ammonium sulphate solution and warm the contents - White precipitate is formed. Inference: The white precipitate is due to the formation of strontium sulphate, SrSO4. Presence of Sr2+ is confirmed. | 1. Ammonium oxalate test: To third part of the above solution in a test tube, add 1 ml of drops of ammonium oxalate solution. Add a little ammonium hydroxide to it and scratch the sides of the test tube with a glass rod - White precipitate is formed. Inference: The white precipitate is due to the formation of calcium oxalate, CaC2O4. Presence of Ca2+ is confirmed. |
2. Flame test: Take a small amount of the salt in a watch glass and add few drops of conc. HCl. Mix the contents well to make a paste. Dip a cleaned platinum wire into this paste and introduce the wire into the non-luminous flame. Note the colour imparted on the flame -Grassy green flame. Inference:Presence of Ba2+ is confirmed. | 2. Flame test: Take a small amount of the salt in a watch glass and add few drops of conc. HCl. Mix the contents well to make a paste. Dip a cleaned platinum wire into this paste and introduce the wire into the non-luminous flame. Note the colour imparted on the flame - Crimson red flame Inference:Presence of Sr2+ is confirmed. | 2. Flame test: Take a small amount of the salt in a watch glass and add few drops of conc. HCl. Mix the contents well to make a paste. Dip a cleaned platinum wire into this paste and introduce the wire into the non-luminous flame. Note the colour imparted on the flame - Brick red flame. Inference:Presence of Ca2+ is confirmed. |
Note:
- Proceed to test for group V cations in the order, Ba2+, Sr2+, Ca2+. If Ba2+ is confirmed do not test for Sr2+ or Ca2+. Similarly if Sr2+ is confirmed, do note test for Ca2+.
- Original solution can be perferably used for testing Sr2+ and Ca2+.
Analysis of Group VI (Magnesium Group)
Experiment | Observation | Inference |
1. Ammonium Phosphate test: To a part of the original salt solution in a test tube, add some solid NH4Cl and solution of NH4OH in slight excess. Then add ammonium phosphate solution and rub the sides of the test-tube with a glass rod. | A white precipitate is formed. | The white precipitate is due to the formation of magnesium ammonium phosphate, Mg(NH4)PO4. Presence of Mg2+ is confirmed. |
2. Cobalt nitrate/Charcoal cavity test: Take a charcoal box with a small cavity in it. Take a small amount of salt in a wach glass. Add solid sodium carbonate whose quantity is 2 time that of the salt into the cavity. Put this mixture in the cavity. Add a drop of water to the mixture. Then direct the reducing flame of Bunsen burner on the cavity by means of blowpipe. Heat strongly for sometime - A white residue is formed. Put one or two drops of cobalt nitrate solution on the white residue left after the cavity in the charcoal cavity test. Direct oxidizing flame into the mixture using blow pipe and observe the colour of the residue - | Pink mass is formed. | Pink mass is due to the formation of MgO.CoO. Presence of Mg2+ is confirmed. |
You can select the Preliminary test from “Select the preliminary test” drop down list.
Colour Test
- Note down the color of original salt.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Smell Test
- Drag the original salt to the watch glass to put it into the watch glass.
- Drag the dropper to the watch glass to drop water into the sample.
- Drag the hand to the watch glass to rub the pre-wetted salt with the fingers.
- If ammoniacal smell, ammonium ion is present.
- Click on the inference icon to see the inference.
- If no characteristics smell, you can go to the next test.
- To redo the test, click on the ‘Reset’ button.
Dry Heating Test
- Drag the original salt to the test tube to put it into the test tube.
- Click on the knob of the burner to turn it on.
- Wait for some time to cool the contents of the test tube.
- Click on the inference icon to see the inference.
- If no characteristics change, you can go to the next test.
- To redo the test, click on the ‘Reset’ button.
Charcoal Cavity Test
- Drag the spatula to the watch glass to add sodium carbonate into the original salt.
- Drag the glass rod to the watch glass to mix the contents of the watch glass.
- Drag the charcoal box near the watch glass to place it in the table.
- Drag the spatula to the watch glass to take the mixture.
- Drag the spatula to the charcoal box to put the mixture in the cavity.
- Drag the bottle to the charcoal box to add water into the mixture.
- Drag the tongs to the charcoal box to hold the box.
- Click on the knob of the burner to turn it on.
- Click on the air adjusting disc to close the air hole to get the reducing flame.
- Drag the tongs to the burner to place the charcoal box near the flame.
- Drag the blow pipe to the burner to direct the reducing flame and heat the mixture.
- Click on the inference icon to see the inference.
- Drag the tongs to the watch glass to return the charcoal box to table and cool the residue.
- Click on the inference icon to see the inference.
- If no characteristics change, you can go to the next test.
- To redo the test, click on the ‘Reset’ button.
Cobalt Nitrate Test
- Drag the dropper to the charcoal box to add cobalt nitrate solution into the residue left in the charcoal box.
- Drag the tongs to the charcoal box to hold the box.
- Click on the knob of the burner to turn it on.
- Drag the tongs to the burner to place the charcoal box near the flame.
- Drag the blow pipe to the burner to direct the oxidizing flame and heat the mixture.
- Click on the inference icon to see the inference.
- If no characteristics change, you can go to the next test.
- To redo the test, click on the ‘Reset’ button.
Flame Test
- Drag the original salt to the watch glass to put it into the watch glass.
- Drag the dropper to the watch glass to pour concentrated Hydrochloric acid into the sample.
- Drag the glass rod to the watch glass to mix the contents well and to make a paste.
- Click on the knob of the burner to turn it on.
- Drag the platinum wire to watch glass to dip it into the paste and drag it to the Bunsen burner to introduce it into the non-luminous flame.
- Click on the inference icon to see the inference.
- If no characteristics change, you can go to the next test.
- To redo the test, click on the ‘Reset’ button.
Borax Bead Test
- Drag the watch glass containing borax to the original salt to place it near the salt.
- Drag the platinum wire to the watch glass to dip it in the borax powder.
- Click on the knob of the burner to turn it on.
- Drag platinum wire to the burner to heat the borax powder in the oxidizing flame.
- Click on the inference icon to see the inference.
- Drag platinum wire to the watch glass to dip it in the original salt.
- Click on the knob of the burner to turn it on to get the oxidizing flame/ Click on the air adjusting disc to close the air hole to get the reducing flame.
- Drag platinum wire to the burner to heat the contents in the oxidizing/reducing flame.
- Click on the inference icon to see the inference.
- Wait for some time to cool the bead on the platinum wire loop.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
You can select the group analysis test from “Select group analysis” drop down list.
Group zero
- Drag the original salt to the test tube to drop it into the test tube.
- Drag the dropper to the test tube to drop NaOH into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- To redo the analysis, click on the ‘Reset’ button.
Group I
- Drag the dropper to the test tube to drop the salt solution into it.
- Drag the dropper to the test tube to drop dil. HCl into the test tube.
- Click on the 'Next' button to go to the next step.
- Drag the dropper to the test tube to drop water into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- To redo the analysis, click on the ‘Reset’ button.
Group II
- Drag the dropper to the test tube to drop dil. HCl into it.
- Click on the knob of the burner to turn it on.
- Click on the ‘Next’ button to go to the next process.
- Drag the test tube to the Kipp’s apparatus to pass H2S gas through the solution.
- Click on the nozzle to open/close the Kipp’s apparatus.
- Click on the inference icon to see the inference.
- Click on the ‘Next’ button to go to the next process.
- To redo the analysis, click on the ‘Reset’ button.
Group III
- Drag the dropper to the test tube to drop Conc.HNO3 into it.
- Click on the knob of the burner to turn it on.
- Drag ammonium chloride (NH4Cl) to the test tube to put it into the test tube.
- Click on the knob of the burner to turn it on.
- Drag the test tube holder to the test tube to hold the test tube.
- Drag the test tube holder to the beaker to place the test tube in water to cool the solution.
- Drag the test tube holder to remove the test tube from the beaker.
- Drag the dropper to the test tube to drop NH4OH into it.
- Click on the test tube to shake it well.
- Click on the inference icon to see the inference.
- To redo the analysis, click on the ‘Reset’ button.
Group IV
- Drag the dropper to the test tube to drop Conc.HNO3 into it.
- Click on the knob of the burner to turn it on.
- Drag ammonium chloride (NH4Cl) to the test tube to put it into the test tube.
- Click on the knob of the burner to turn it on.
- Drag the test tube holder to the test tube to hold the test tube.
- Drag the test tube holder to the beaker to place the test tube in water to cool the solution.
- Drag the test tube holder to remove the test tube from the beaker.
- Drag the dropper to the test tube to drop NH4OH into it.
- Click on the test tube to shake it well.
- Click on the ‘Next’ button to go to the next process.
- Drag the test tube to the Kipp’s apparatus to pass H2S gas through the solution.
- Click on the nozzle to open/close the Kipp’s apparatus.
- Click on the inference icon to see the inference.
- To redo the analysis, click on the ‘Reset’ button.
Group V
- Drag ammonium chloride (NH4Cl) to the test tube to put it into the test tube.
- Click on the knob of the burner to turn it on.
- Wait for some time to cool the solution.
- Drag the dropper to the test tube to drop ammonium hydroxide solution into it.
- Drag the dropper to the test tube to drop ammonium carbonate solution into it.
- Click on the inference icon to see the inference.
- Click on the ‘Next’ button to go to the next process.
- Drag the dropper to the test tube to drop hot dil. CH3COOH (acetic acid) into it.
- Click on the inference icon to see the inference.
- Click on the ‘Next’ button to go to the next process.
- To redo the analysis, click on the ‘Reset’ button.
You can select the cation from ‘Select the cation’ drop down list.
You can select the confirmatory tests from ‘Select the confirmatory tests’ drop down list.
Group Zero- Ammonium<
Sodium Hydroxide Test
- Drag the original salt to the test tube to drop it into the test tube.
- Drag the dropper to the test tube to drop NaOH into it.
- Click on the knob of the burner to turn it on.
- Drag the glass rod to the bottle to dip it in dil. HCl and drag it to the test tube to hold it over the mouth of the test tube.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Nessler’s Reagent Test
- Drag the original salt to the test tube to drop it into the test tube.
- Drag the dropper to the test tube to drop NaOH into it.
- Click on the knob of the burner to turn it on.
- Drag the cork to the test tube to close the test tube with it.
- Drag the delivery tube to the test tube to insert it between the test tube and Nessler’s reagent.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Group (I) - Lead (II)
Cool the Solution
- Drag the dropper to the test tube to drop water into it.
- Click on the knob of the burner to turn it on.
- To cool the contents of the test tube, drag the test tube to the beaker and place it in the beaker containing cold water.
- Drag the test tube to remove it from the beaker.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Potassium Iodide Test
- Drag the dropper to the test tube to drop potassium iodide solution into it.
- Potassium Chromate Test
- Drag the dropper to the test tube to drop potassium chromate solution into it.
Group (II) - Lead (II)
Potassium Iodide Test
- Drag the dropper to the test tube to drop 50% HNO3 into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop dil. H2SO4 into it.
- Drag the dropper to the test tube to drop ethyl alcohol (C2H5OH) into it.
- Click on the inference icon to see the inference.
- Click on the ‘Next’ button to go to the next process.
- Drag the dropper to the test tube to drop hot ammonium acetate solution into the test tube.
- Drag the dropper to the test tube to drop potassium iodide solution into it.
- Click on the knob of the burner to turn it on.
- Drag the test tube to the beaker to place it in the beaker containing water and cool it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Potassium Chromate Test
- Drag the dropper to the test tube to drop 50% HNO3 into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop dil. H2SO4 into it.
- Drag the dropper to the test tube to drop ethyl alcohol (C2H5OH) into it.
- Click on the inference icon to see the inference.
- Click on the ‘Next’ button to go to the next process.
- Drag the dropper to the test tube to drop hot ammonium acetate solution into it.
- Drag the dropper to the test tube to drop potassium chromate solution into it.
- Drag the dropper to the test tube to drop NaOH solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Group (II) - Copper (II)
Potassium Ferrocyanide Test
- Drag the dropper to the test tube to drop 50% HNO3 into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop dil. H2SO4 into it.
- Drag the dropper to the test tube to drop ethyl alcohol (C2H5OH) into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop ammonium hydroxide solution into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop acetic acid into it.
- Drag the dropper to the test tube to drop potassium ferrocyanide solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Potassium Iodide Test
- Drag the dropper to the test tube to drop 50% HNO3 into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop dil. H2SO4 into it.
- Drag the dropper to the test tube to drop ethyl alcohol (C2H5OH) into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop ammonium hydroxide solution into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop acetic acid into it.
- Drag the dropper to the test tube to drop potassium iodide solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Group (II) - Arsenic (III)
Ammonium Molybdate Test
- Drag the dropper to the test tube to drop Conc. HNO3 into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop ammonium molybdate solution into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Magnesia Mixture Test
- Drag the dropper to the test tube to drop Conc. HNO3 into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop NH4OH solution into it.
- Drag the dropper to the test tube to drop Magnesia mixture into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Group (III) - Iron (III)
Potassium Ferrocyanide Test
- Drag the dropper to the test tube to drop dil. HCl into the test tube.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop Potassium ferrocyanide solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Potassium Sulphocyanide Test
- Drag the dropper to the test tube to drop dil. HCl into the test tube.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop Potassium sulphocyanide solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Group (III) - Aluminium (III)
Lake Test
- Drag the dropper to the test tube to drop dil. HCl into it.
- Drag the dropper to the test tube to drop blue litmus solution into it.
- Drag the dropper to the test tube to drop NH4OH solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Charcoal Cavity Test/ Cobalt Nitrate Test
- Refer to the procedure for Charcoal Cavity Test/ Cobalt Nitrate Test in the ‘Preliminary Tests’.
Group (IV) - Cobalt (II)
Potassium Nitrite Test
- Drag the dropper to the test tube to drop NH4OH into it.
- Drag the dropper to the test tube to drop acetic acid into it.
- Drag the spatula to the test tube to put potassium nitrite crystals into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Ammonium thiocyanate - Ether Test
- Drag the dropper to the test tube to drop ether into it.
- Drag the spatula to the test tube to add ammonium thiocyanate into it.
- Drag the test tube from the stand to shake it well.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Borax bead Test
- Refer to the procedure for Borax Bead Test in the ‘Preliminary Tests’.
Group (IV) - Nickel (II)
Dimethyl glyoxime Test
- Drag the dropper to the test tube to drop NH4OH solution into it.
- Drag the dropper to the test tube to drop dimethyl glyoxime into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Sodium hydroxide – Br2 Test
- Drag the dropper to the test tube to drop NaOH into it.
- Drag the dropper to the test tube to drop bromine water into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Borax Bead Test
- Refer to the procedure for Borax Bead Test in the ‘Preliminary Tests’.
Group (IV) - Manganese (II)
Sodium hydroxide – Br2 Test
- Drag the dropper to the test tube to drop dil. HCl into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop NaOH into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop bromine water into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Lead Peroxide Test
- Drag the dropper to the test tube to drop Conc. HNO3 into it.
- Drag the spatula to the test tube to add lead peroxide into it.
- Click on the knob of the burner to turn it on.
- Wait for some time to cool the solution.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Borax Bead Test
- Refer to the procedure for Borax Bead Test in the ‘Preliminary Tests’.
Group (IV) - Zinc (II)
Sodium Hydroxide Test
- Drag the dropper to the test tube to drop NaOH solution into it.
- Click on the inference icon to see the inference.
- Drag the dropper to the test tube to drop more NaOH solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Potassium Ferrocyanide Test
- Drag the dropper to the test tube to drop potassium ferrocyanide solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Charcoal Cavity Test/ Cobalt Nitrate Test
- Refer to the procedure for Charcoal Cavity Test/ Cobalt Nitrate Test in the ‘Preliminary Tests’.
Group (V) - Barium (II)
Potassium Chromate Test
- Drag the dropper to the test tube to drop potassium chromate solution into it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Flame Test
- Refer to the procedure for Flame Test in the ‘Preliminary Tests’.
Group (V) - Strontium (II)
Ammonium Sulphate Test
- Drag the dropper to the test tube to drop ammonium sulphate solution into it.
- Click on the knob of the burner to turn it on.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Flame Test
- Refer to the procedure for Flame Test in the ‘Preliminary Tests’.
Group (V) - Calcium (II)
Ammonium Oxalate Test
- Drag the dropper to the test tube to drop ammonium oxalate solution into it.
- Drag the dropper to the test tube to drop ammonium hydroxide solution into it.
- Drag the glass rod to the test tube to rub the sides of the test tube with it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Flame Test
- Refer to the procedure for Flame Test in the ‘Preliminary Tests’.
Group (VI) – Magnesium (II)
Ammonium Phosphate Test
- Drag ammonium chloride (NH4Cl) to the test tube to put it into the test tube.
- Drag the dropper to the test tube to drop excess NH4OH into it.
- Drag the dropper to the test tube to drop ammonium phosphate [(NH4)3PO4] solution into it.
- Drag the glass rod towards the test tube to rub the sides of the test tube with it.
- Click on the inference icon to see the inference.
- To redo the test, click on the ‘Reset’ button.
Charcoal Cavity Test/ Cobalt Nitrate Test
- Refer to the procedure for Charcoal Cavity Test/ Cobalt Nitrate Test in the ‘Preliminary Tests’.
Note: Click on the 'HELP' button to see the instructions.
Precautions
- Handle the apparatus and chemicals with care.
- When heating a solution in a test tube, students should hold the test tube with a proper holder.
- While heating, the mouth of the test tube should not point towards the student or any other person in the lab.
- Students should wear lab coats and goggles while performing the experiment.
|
26 videos|312 docs|64 tests
|
FAQs on Theory & Procedure, Qualitative Analysis of Cations - Additional Study Material for NEET
| 1. What is qualitative analysis of cations? |  |
| 2. Why is qualitative analysis of cations important? |  |
| 3. What is the procedure for qualitative analysis of cations? |  |
| 4. What are the common reagents used in qualitative analysis of cations? |  |
| 5. What are the limitations of qualitative analysis of cations? |  |
















