NEET Exam > NEET Notes > NCERT Exemplar & Revision Notes for NEET > Revision Notes: Thermodynamics
Thermodynamics Class 11 Notes Physics Chapter 11
- Thermodynamics:- It is the branch of physics which deals with process involving heat, work and internal energy. Thermodynamics is concerned with macroscopic behavior rather than microscopic behavior of the system.
- Basic Terminology:-
System | Part of the universe under investigation. |
Open System | A system which can exchange both energy and matter with its surroundings. |
Closed System | A system which permits passage of energy but not mass, across its boundary. |
Isolated system | A system which can neither exchange energy nor matter with its surrounding. |
Surroundings | Part of the universe other than system, which can interact with it. |
Boundary | Anything which separates system from surrounding. |
State variables | The variables which are required to be defined in order to define state of any system i.e. pressure, volume, mass, temperature, surface area, etc. |
State Functions | Property of system which depend only on the state of the system and not on the path. Example: Pressure, volume, temperature, internal energy, enthalpy, entropy etc. |
Intensive properties | Properties of a system which do not depend on mass of the system i.e. Temperature, pressure, density, concentration, |
Extensive properties | Properties of a system which depend on mass of the system i.e. Volume, energy, enthalpy, entropy etc. |
Process | Path along which state of a system changes. |
Isothermal process | Process which takes place at constant temperature |
Isobaric process | Process which takes place at constant pressure |
Isochoric process | Process which takes place at constant volume. |
Adiabatic process | Process during which transfer of heat cannot take place between system and surrounding. |
Cyclic process | Process in which system comes back to its initial state after undergoing series of changes. |
Reversible process | Process during which the system always departs infinitesimally from the state of equilibrium i.e. its direction can be reversed at any moment. |
- Kinetic Energy:- Energy possessed by the atoms or molecules by virtue of their motion is called kinetic energy.
- Internal Energy (ΔU):- Sum total of kinetic and potential energies of atoms/molecules constituting a system is called the internal energy of the system.
(a) ΔU is taken as positive if the internal energy of the system increases.
(b) ΔU is taken as negative if the internal energy of the system decreases. - Heat:- Heat is the part of internal energy which is transferred from one body to another an account of the temperature difference.
- Work:- Work is said to be done when a force acting on a system displaces the body in its own direction.
dW = Fdx = PdV
W = P(Vf -Vi)
(a) If the gas expands, work is said to be done by the system. In this case Vf > Vi, therefore, W will be positive.
(b) If the gas is compressed, work is said to be done on the system. In this case Vf < Vi, therefore, work done is negative. - Thermodynamic variables or parameters:- The thermodynamic state of system can be determined by quantities like temperature (T), volume (V), pressure (P), internal energy (U) etc. These quantities are known as thermodynamic variables, or the parameters of the system.
- Equation of state:- A relation between the values of any of the three thermodynamic variables for the system, is called its equation of state.
Equation of state for an ideal gas is PV = RT - Equilibrium of a system:- A system is said to be in equilibrium if its macroscopic quantities do not change with time.
- Relation between joule and calorie:- 1 joule = 4.186 cal
- First law of thermodynamics:- If the quantity of heat supplied to a system is capable of doing work, then the quantity of heat absorbed by the system is equal to the sum of the increase in the internal energy of the system, and the external work done by it.
dQ = dU+dW - Thermodynamic Process:- A process by which one or more parameters of thermodynamic system undergo a change is called a thermodynamic process or a thermodynamic change.
(a) Isothermal process:- The process in which change in pressure and volume takes place at a constant temperature, is called a isothermal change. It may be noted that in such a change total amount of heat of the system does not remain constant.
(b) Isobaric process:- The process in which change in volume and temperature of a gas take place at a constant pressure is called an isobaric process.
(c) Isochoric process:- The process in which changes in pressure and temperature take place in such a way that the volume of the system remains constant, is called isochoric process.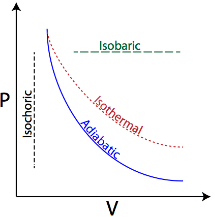
(d) Adiabatic process:- The process in which change in pressure and volume and temperature takes place without any heat entering or leaving the system is called adiabatic change.
(e) Quasi-static process:- The process in which change in any of the parameters take place at such a slow speed that the values of P,V, and T can be taken to be, practically, constant, is called a quasi-static process.
(f) Cyclic process:- In a system in which the parameters acquire the original values, the process is called a cyclic process.
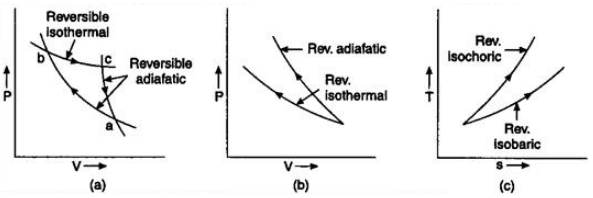
(g) Free expansion:- Such an expansion in which no external work is done and the total internal energy of the system remains constant is called free expansion. - Reversible isothermal and adiabatic curve:-
- Application of first law of thermodynamics:-
(a) Cooling caused in adiabatic process:- dT = PdV/Cv
(b) Melting:- dU = mLf
(c) Boiling:- dU = mLv – P(Vf -Vi)
(d) Mayer’s formula:- Cp - Cv = R - Specific heat capacity of gases:- Specific heat capacity of a substance is defined as the amount of heat required to raise the temperature of a unit mass of substance through 1ºC.
(a) Specific heat capacity at constant volume (cv):- Specific heat capacity at constant volume is defined as the amount of heat required to raise the temperature of 1 g of the gas through 1ºC keeping volume of the gas constant.
Molar specific heat capacity, at constant volume (Cv), is defined as the amount of heat required to raise the temperature of 1 mole of gas through 1ºC keeping its volume constant.
Cv= Mcv
(b) Specific heat capacity at constant pressure (cp):- Specific heat capacity, at constant pressure, is defined as the amount of heat required to raise the temperature of 1 g of gas through 1ºC keeping its pressure constant.
Gram molecular specific heat capacity of a gas (Cp), at constant pressure, is defined as the amount of heat required to raise the temperature of 1 mole of the gas through 1ºC keeping its pressure constant.
Cp = Mcp - Difference between two specific heat capacities – (Mayer’s formula):-
(a) Cp - Cv = R/J
(b) For 1 g of gas, cp - cv = r/J
(c) Adiabatic gas constant, γ = Cp/ Cv = cp/ cv - Relation of Cv with energy:-
Cv= 1/m (dU/dT)
(a) Mono-atomic gas (3 degree of freedom):-
Total energy, U = mN 3 [(1/2) KT], Here m is the number of moles of the gas and N is the Avogadro’s number.
Cv = (3/2) R
Cp = (5/2) R
γ = Cp/ Cv = 5/3 = 1.67
(b) Diatomic gas:-
At very low temperature, Degree of Freedom (DOF) = 3
U = (3/2) mRT
Cv = (3/2) R, Cp = (5/2) R
γ= Cp/ Cv = 5/3 = 1.67
At medium temperature, DOF = 5
U = (5/2) mRT
Cv = (5/2) R, Cp = (7/2) R
γ = Cp/ Cv = 7/5 = 1.4
At high temperature, DOF = 7
U = (7/2) mRT
Cv = (7/2) R, Cp = (9/2) R
γ = Cp/ Cv = 9/7 = 1.29 - Adiabatic gas equation:- PV γ = Constant
(a) Equation of adiabatic change in terms of T and V:- TV γ-1 = Constant
(b) Equation of adiabatic change in terms of P and T:- T γ P1-γ = Constant - Comparison of slopes of an isothermal and adiabatic:-
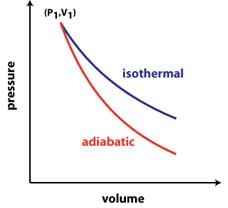
(a) Slope of isothermal:- dP/dV = -P/V
(b) Slope of adiabatic:- dP/dV = -γP/V
(c) Adiabatic gas constant:- γ = Cp/Cv
As, Cp>Cv, So, γ>1
This signifies that, slope of adiabatic curve is greater than that of isothermal. - Slope on PV diagram:-
(a) For isobaric process: zero
(b) For isochoric process: infinite - Work done for isobaric process:- W = P(V2-V1)
- Work done for isochoric process:- W = 0
- Work done in isothermal expansion and compression:-
W = 2.3026 RT log10Vf/Vi (isothermal expansion)
W = - 2.3026 RT log10Vf/Vi (isothermal compression)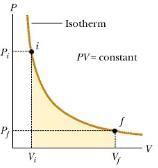
- Work done during an adiabatic expansion:-
W = K/1-γ [Vf1-γ – Vi 1-γ] = 1/1-γ [P2V2-P1V1] = R/1- γ [T2-T1] - Adiabatic constant (γ):- γ = Cp/Cv = 1+2/f, Here f is the degrees of freedom.
- Work done in expansion from same initial state to same final volume:-
- Wadiabatic < Wisothermal < Wisobaric
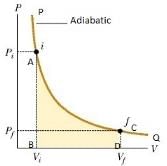
- Work done in compression from same initial state to same final volume:-
Wadiabatic < Wisothermal < Wisobaric - Reversible process:- It is a process which can be made to proceed in the reverse direction by a very slight change in its conditions so that the system passes through the same states as in direct process, and at the conclusion of which the system and its surroundings acquire the initial conditions.
Example:- All isothermal and adiabatic process when allowed to proceed slowly, are reversible, provided there is no loss of energy against any type of resistance. Friction, viscosity are other examples. - Irreversible process:- A process which cannot be made to be reversed in opposite direction by reversing the controlling factor is called an irreversible process.
Example:-
(a) work done against friction
(b) Joule’s heating effect
(c) Diffusion of gases into one another
(d) Magnetic hysteresis - Heat engine:- It is a device used to convert heat into mechanical energy
(a) Work done, W = Q1-Q2
(b) Efficiency:- Efficiency η of an engine is defined as the fraction of total heat, supplied to the engine which is converted into work.
η= W/ Q1 = [Q1- Q2]/ Q1 = 1-[Q2/Q1] - Carnot engine – Carnot’s reverse cycle:-
(a) First stroke (isothermal expansion):- W1= RT1 loge[V2/V1]
(b) Second stroke (adiabatic expansion):- W2= R/γ-1 [T1-T2]
(c) Third stroke (isothermal compression):- W3= RT2 logeV3/V4
(d) Fourth stroke (adiabatic compression):- W4= R/γ-1 [T1-T2]
(e) Total work done in one cycle, W = W1+ W2+ W3+ W4 = R (T1-T2) loge (V2/V1) - Efficiency of Carnot engine:- Efficiency η of an engine is defined as the ratio between useful heat (heat converted into work) to the total heat supplied to the engine.
η = W / Q1 = [Q1- Q2]/ Q1 = 1-[Q2/Q1] = 1- T2/T1 - Second law of thermodynamics:-
(a) Clausius statement:- Heat cannot flow from a cold body to a hot body without the performance of work by some external agency.
(b) Kelvin’s statement:- It is impossible to obtain a continuous supply of energy by cooling a body below the coldest of its surroundings.
(c) Planck’s statement:- It is impossible to extract heat from a single body and convert the whole of it into work. - Refrigerator:- It is a device which is used to keep bodies at a temperature lower than that of surroundings.
- Coefficient of performance (β):- Coefficient of performance of a refrigerator is defined as the amount of heat removed per unit work done on the machine.
β = Heat removed/work done = Q2/W = Q2/[Q1- Q2] = T2/[T1- T2]
Coefficient of performance of a refrigerator is not a constant quantity since it depends upon the temperature of body from which the heat is removed.
For a perfect refrigerator, W = 0 or Q1= Q2 or β =∞ - Mean free path:- λ= 1/√2πd2ρn
Here ρn = (N/V) = number of gas molecules per unit volume
d = diameter of molecules of the gas. - Heat added or removed:-
(a) For isobaric process:- Q = n CpΔT
(b) For isochoric process:- Q = n CvΔT
(c)For isothermal process:- Q = nRT loge (V2/V1)
(d) For adiabatic process: Q = 0 - Change in internal energy:-
(a) For isobaric process, ΔU = n CpΔT
(b) For isobaric process, ΔU = n CvΔT
(c) For isothermal process, ΔU = 0
(d) For adiabatic process, ΔU = -W = [nR (T2-T1)]/(γ-1) - Mixture of gases:- n = n1+n2
M = n1M1+n2M2/ n1+ n2 = N1m1+N2m2/N1+N2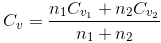
and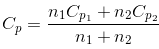
- Enthalpy (H):-
(a) H = U+PV
(b) At constant pressure:-
dH = dU + pdV
(c) For system involving mechanical work only:-
dH = QP (At constant pressure)
(d) For exothermic reactions:-
dH is negative
(e) For endothermic reactions:-
dH is positive - Relation between dH and dU:-
dH = dU + dng RT
Here, dng = (Number of moles of gaseous products - Number of moles of gaseous reactants
The document Thermodynamics Class 11 Notes Physics Chapter 11 is a part of the NEET Course NCERT Exemplar & Revision Notes for NEET.
All you need of NEET at this link: NEET
FAQs on Thermodynamics Class 11 Notes Physics Chapter 11
| 1. What is thermodynamics? |  |
Ans. Thermodynamics is the branch of physics that deals with the study of heat, energy, and their interconversion. It involves understanding how energy is transferred between different forms, such as heat and work, and how it affects the behavior of systems.
| 2. What are the laws of thermodynamics? |  |
Ans. The laws of thermodynamics are fundamental principles that govern the behavior of energy and heat transfer in systems. The three main laws are:
1. The first law of thermodynamics, also known as the law of energy conservation, states that energy cannot be created or destroyed, only transferred or transformed.
2. The second law of thermodynamics states that the total entropy of an isolated system always increases over time. Entropy is a measure of the system's disorder or randomness.
3. The third law of thermodynamics states that as the temperature approaches absolute zero, the entropy of a pure crystalline substance also approaches zero.
| 3. What is the difference between heat and work in thermodynamics? |  |
Ans. Heat and work are both forms of energy transfer, but they differ in how they are transferred. Heat is the transfer of energy due to a temperature difference between two objects, while work is the transfer of energy due to a force acting on an object and causing it to move.
In thermodynamics, heat is denoted as Q and work as W. Heat is transferred across a temperature gradient, while work is done by applying a force to move an object. Both heat and work can change the internal energy of a system.
| 4. How does thermodynamics relate to everyday life? |  |
Ans. Thermodynamics has various practical applications in everyday life. Some examples include:
1. Household appliances: Thermodynamics is involved in the functioning of refrigerators, air conditioners, and heaters. These appliances transfer heat from one place to another to maintain a desired temperature.
2. Cooking: Thermodynamics plays a role in cooking processes, such as boiling water, baking, and grilling. Understanding heat transfer and energy conversion helps in achieving desired cooking results.
3. Power generation: Thermodynamics is essential in power plants that generate electricity. It involves converting heat energy from burning fuels, such as coal or gas, into mechanical energy, which is then transformed into electrical energy.
4. Automotive engines: The efficiency of car engines is influenced by thermodynamic principles. Combustion processes, heat transfer, and energy conversion are crucial for optimizing fuel consumption and engine performance.
| 5. What is the significance of thermodynamics in chemical reactions? |  |
Ans. Thermodynamics is crucial in understanding and predicting the behavior of chemical reactions. It helps determine whether a reaction is spontaneous or requires an external energy input.
Thermodynamics provides information about the direction of a reaction, the equilibrium state, and the maximum work that can be obtained from a reaction. It also helps in calculating the heat and energy changes during a reaction, which are essential for designing efficient chemical processes.
Related Searches
















