d & f-Block Elements Class 12 Notes Chemistry Chapter 4
Physicochemical Properties
a. Melting and Boiling Points: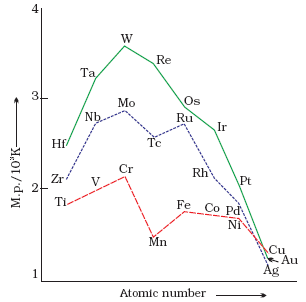
Melting and boiling points show no definite trends in the three transition series.
The metals having the highest melting and boiling points are towards the middle of each transition series.
b. Atomic (Covalent) and Ionic Radii:
Atomic and ionic radii values decrease generally, on moving from left to right in the period.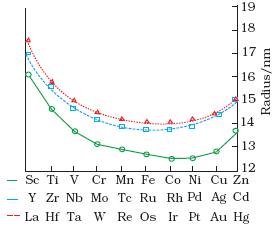
The atomic radii for the elements from Cr to Cu are very close to one another.
Radii of 5d series elements are virtually the same as those of corresponding members of 4d series due to lanthanoid contraction.
c. Ionisation Potentials:
- First Ionization Potentials: I1 values for the first four 3d block elements (Sc, Ti, V and Cr) differ only slightly from one another. The value of II for Zn is considerably higher. This is due to the extra-stability of 3d10 level which is completely filled in Zn-atom.
- Second ionisation potentials: The value of III for Cr and Cu are higher than those of their neighbours. This is due to the fact that the electronic configurations of Cr+ and Cu+ ions have extra stable 3d5 and 3d10 levels.There is a sudden fall in the values of ionisation potentials in going from II B (Zn-group elements) to IIIA sub-group.
d. Oxidation States: - The higher oxidation state of 4d and 5d series elements are generally more stable than those of the elements of 3d series,
- In short it may be said that in going down a sub-group the stability of the higher oxidation states increases while that of lower oxidation states decreases.
- Transition elements cannot form ionic compounds in higher oxidation states because the loss of more than three electrons is prevented by the higher attractive force exerted (on the electrons) by the nucleus
e. Colour: Transition elements with partially filled d orbitals form coloured compounds.
f. Complex Formation: Transition elements show tendency to form complex compounds due to. - Small size and high effective nuclear charge
- Availability of low lying vacant d–orbitals which can accept lone pair of electrons donated by a ligand.
g. Catalytic properties:
Transition metals and their compounds are known to act as good catalyst due to
1. variable oxidation state, they form unstable intermediate compounds and provide a new path with lower activation energy for the reaction (Intermediate compound formation theory)
2. In some cases the finely divided metals or their compounds provide a large surface area for adsorption and the adsorbed reactants react faster due to the closer contact(Adsorption theory)
h. Magnetic Properties:
Magnetic moment is which is related to the number of unpaired electrons as follows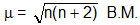
n = number of unpaired electrons
B.M. = Bohr Magneton, unit of magnetic moment
More the magnetic moment more is the paramagnetic behaviour
i. Formation of Alloys:
As the transition elements have similar atomic sizes hence in the crystal lattice, one metal can be readily replaced by another metal giving solid solution and smooth alloys. The alloys so formed are hard and have often high melting point.
j. Interstitial Compounds:
Transition metals form no. of interstitial compounds, in which they take up atoms of small size e.g. H, C and N in the vacant spaces in the their lattices. The presence of these atoms results in decrease in malleability and ductility of the metals but increases their tensile strength.
Potassium Dichromate (K2Cr2O7)
a. Preparation
It is prepared from the ore called chromate or ferrochrome or chrome iron, FeO.Cr2O3.
Steps 1: Preparation of sodium chromat
4FeO.Cr2O3 + O2 → Fe2O3 + 4Cr2O3
4Na2CO3 + 2Cr2O3 + 3O2 → 4Na2CrO4 + 4CO2
Step 2: Conversion of sodium chromate into sodium dichromate.
2Na2CrO4 + H2SO4 → Na2Cr2O7 + Na2SO4 + H2O
Step 3: Conversion of sodium dichromate into potassium dichromate.
Na2Cr2O7 + 2KCl → K2Cr2O7 + 2NaCl
b. Properties
1. Action of heat: When heated, it decomposed to its chromate
4K2Cr2O7 +Δ→ 4K2CrO4 + 2Cr2O3 + 3O2
2. Action of alkalis
K2Cr2O7 + 2KOH → 2K2CrO4 + H2O
2K2Cr2O7 + H2SO4 → K2Cr2O7 + K2SO4 + H2O
3. Action of conc. H2SO4 solution
(a) In cold conditions
K2Cr2O7 + 2H2SO4 → 2CrO3 + 2KHSO4 + H2O
(b) In hot conditions
2K2Cr2O7 + 8H2SO4 → 2K2SO4 + 2Cr2(SO4)3 + 8H2O + 3O2
4. Oxidising propertiesIt is a powerful oxidising agent.
In the presence of dil. H2SO4 it furnishes 3 atoms of available oxygen.
K2Cr2O7 + 4H2SO4 → K2SO4 + Cr2(SO4)3 + 4H2O + 3O
Some of the oxidizing properties of K2Cr2O7 are
It liberates I2 from KI
K2Cr2O7 + 7H2SO4 + 6Kl → 4K2SO4 + Cr2(SO4)3 + 3l2 + 7H2O
It oxidises ferrous salts to ferric salts
K2Cr2O7 + 7H2SO4 + 6FeSO4 → K2SO4 + Cr2(SO4)3 + 3Fe2(SO4)3 + 2H2O
It oxidises S-2 to S
K2Cr2O7 + 4H2SO4 + 3H2S → K2SO4 + Cr2(SO4)3 + 7H2O + 3S
It oxidises nitrites to nitrates
K2Cr2O7 + 4H2SO4 + 3NaNO2 → K2SO4 + Cr2(SO4)3 + 3NaNO3 + 4H2O
It oxidises SO2 to SO42–
K2Cr2O7 + H2SO4 + 3SO2 → K2SO4 + Cr2(SO4)3 + 3H2O
It oxidises ethyl alcohol to acetaldehyde and acetic acid.
5. Chromyl chloride test
When heated with conc. HCl or with a chloride in the presence of sulphuric acid, reddish brown vapours of chromyl chloride are obtained.
K2Cr2O7 + 4KCl + 6H2SO4 → 2CrO2Cl2 + 6KHSO4 + 3H2O
Thus reaction is used in the detection of chloride ions in qualitative analysis.
c. Uses
In volumetric analysis for the estimation of Fe2+ and I-.
In chrome tanning in leather industry.
In photography and in hardening gelatin film.
Potassium Permanganate
a. Preparation:
It is prepared from the mineral pyrolusite, MnO2.
Step 1: Conversion of MnO2 into potassium manganate.
2MnO2 + 4KOH + O2 → 2K2MnO4 + 2H2O
Step 2: Oxidation of potassium manganate into permanganate
Chemical oxidation
K2MnO4 is oxidised to KMnO4 by bubbling CO2 or Cl2 or ozone into the former.
3K2MnO4 + 2CO2 → 2KMnO4 + MnO2 + 2K2CO3
Electrolytic oxidation
2K2MnO2 + H2O + O → 2K2MnO4 + 2KOH
b. Properties
KMnO4 exists as deep purple prisms. It is moderately soluble in water at room temperature and its solubility in water increases with temperature.
(i) Action of heat
When heated it decomposes to K2MnO4.
2KMnO4 → K2MnO4 + MnO2 + O2
(ii) Action of conc. H2SO4
With cold conc. H2SO4 it gives Mn2O7 which on warming decomposes to MnO2.
2MnO2 + 2H2SO4 → Mn2O7 + 2KHSO4 + 2H2O
2Mn2O7 + Δ → 4MnO2 + 3O2
With hot Conc. H2SO4 O2 is evolved
4KMnO4 + 6H2SO4 → 2K2SO4 + 4MnSO4 + 6H2O + 5O2
(iii) Oxidising properties
KMnO4 is a powerful oxidizing agent. The actual oxidizing action depends upon themedium i.e. acidic, basic or neutral.
(a) In neutral solution, it acts as moderate oxidizing agent.
2KMnO4 + H2O → 2KOH + 2MnO2 + 3O
Some oxidizing properties of KMnO4 in neutral medium are
2KMnO4 + 3Na2S2O3 + H2O → 3K2SO4 + 8MnO2 + 3Na2SO4 + 2KOH
2KMnO4 + 4H2S → 2MnS + S + K2SO4 + 4H2O
(b) In strong alkaline solution, it is converted into
2KMnO4 + 2KOH → 2K2MnO4 + H2O + O
Some reaction in alkaline medium are
2KMnO4 + H2O + Kl → 2MnO2 + 2KOH + KlO3
(c) In acidic medium, Mn+7 is converted into Mn+2
2KMnO4 + 3H2SO4 → K2SO4 + 2MnSO4 + 3H2O + 5O
Some other reactions are
(i) 2KMnO4 + 3H2SO4 + 5H2S → K2SO4 + 2MnSO4 + 3H2O + 5S
(ii) 2KMnO4 + 5SO2 + 2H2O → K2SO4 + 2MnSO4 + 2H2SO4
(iii) 2KMnO4 + 3H2SO4 + 5KNO2 → K2SO4 + 2MnSO4 + 3H2O + 5KNO3
(iv) 2KMnO4 + 3H2SO4 + 5C2H2O4 → K2SO4 + 2MnSO4 + 8H2O + 10CO2
(v) 2KMnO4 + 8H2SO4 + 10FeSO4 → K2SO4 + 2MnSO4 + 5Fe2(SO4)3 + 8H2O
(vi) 2KMnO4 + 3H2SO4 + 10Kl → K2SO4 + 2MnSO4 + 8H2O + 5l2
c. Uses
(i)Used in volumetric analysis for estimation of ferrous salts, oxalates, iodides & H2O2.
(ii) Used as oxidizing agent in the laboratory as well as in industry.
(iii) Used as disinfectant and germicide.
Inner Transition Elements
The f-block elements are known as inner transition elements because they involve the filling for inner sub-shells (4f or 5f)
a. Lanthanides:
It consists of elements that follows lanthanum and involve the filling of 4 subshell
Electronic Configuration : [Xe] 4fn+1 5d° 6s2 or [Xe] 4fn 5d1 6s2
Oxidation State: +3, +2 and +4.
Colouration: Many of the lanthanides ions are coloured in solid state as well as in solutions. The colour is due to the f-f transition since they have partly filled f-orbitals.
Lanthanide Contraction: The steady decrease in the size of lanthanide ions (M3+) with the increase in atomic no. is called lanthanide contraction.
Causes: As we move down the group from left to right in a lanthanide series, the atomic no. increases and for every proton in the nucleus the extra electron goes to 4f orbital. The 4f orbital is too diffused to shield the nucleus effectively, thus there is a gradual increase in the effective nuclear charge experienced by the outer electrons. Consequently , the attraction of the nucleus for the electrons in the outermost shell increases with the increase of atomic number, thus size decreases.
Consequence of Lanthanide Contraction:
Separation of Lanthanides: Due to the similar sizes of the lanthanides, it is difficult to separate them but due to lanthanide contraction their properties slightly vary (such as ability to form complexes). The variation in the properties is utilized to separate them.
Basic Strength of Hydroxide: Due to the lanthanide contraction, size of M3+ ions decreases and there is increase in covalent character in M–OH and hence basic character decreases.
Similarity of second and third transition series: The atomic radii of second row transition elements are almost similar to those of the third row transition elements because the increase in size on moving down the group from second to third transition elements is cancelled by the decrease in size due to the lanthanide contraction
b. Actinides:
It consists of elements that follow Actinium and involve the filling of 5f subshell.
These are radioactive substances.
7s2 is stable configuration for actinides.
Show +3,+4,+5,+6 & +7 oxidation state.
Lower ionization enthalpies than lanthinoids.
FAQs on d & f-Block Elements Class 12 Notes Chemistry Chapter 4
| 1. What are d-block elements? |  |
| 2. What are f-block elements? |  |
| 3. Why are d-block elements called transition elements? |  |
| 4. What are the properties of d-block elements? |  |
| 5. What are some applications of d-block elements? |  |

















