Atomic Number & Mass Number, Isotopes & Isobars | Chemistry Class 11 - NEET PDF Download
| Table of contents |

|
| Atomic Number (Z) |

|
| Mass Number (A) |

|
| Representation of an Atom |

|
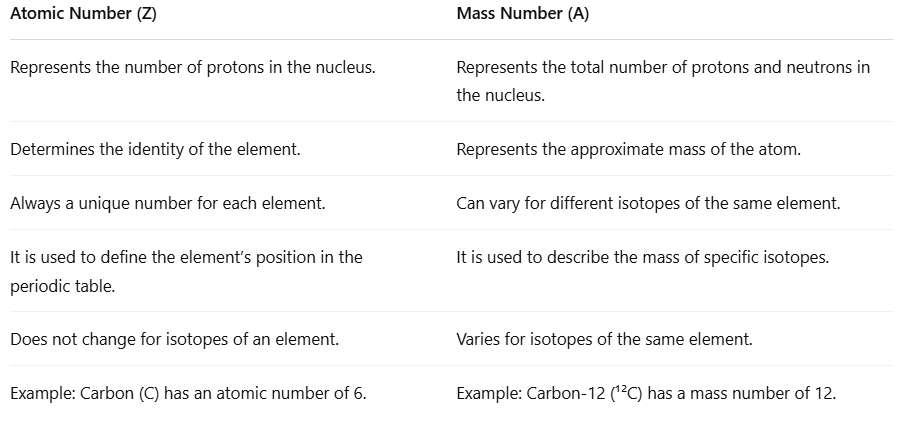
| Difference Between Atomic Number and Mass Number |

|
| Different Types of Atomic Species |

|
| Solved Questions |

|
Atomic Number (Z)
The atomic number is the number of protons found in the nucleus of an atom of a given element. Since protons carry a positive charge, the atomic number also determines the positive charge of the nucleus.
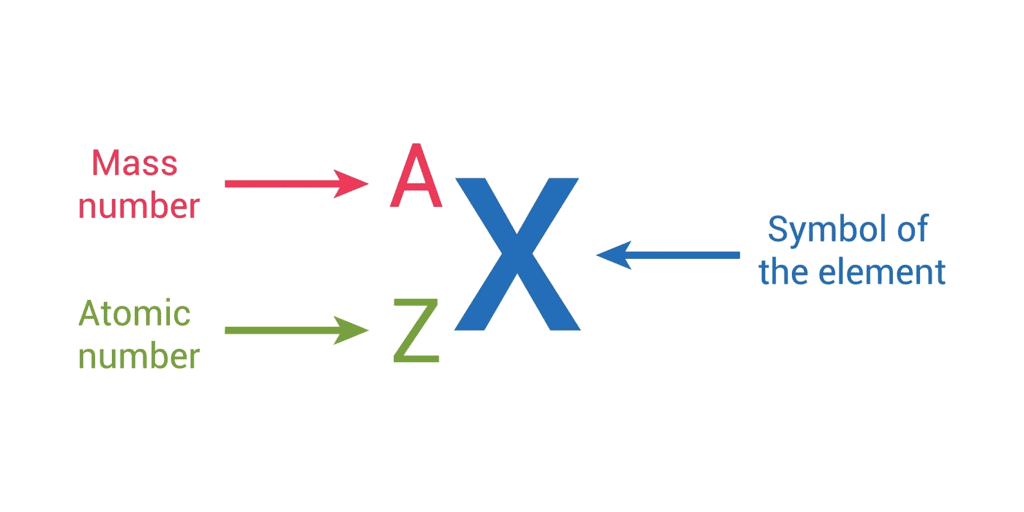
- It is represented with the letter ‘Z.’
- All the atoms of a particular element have the same number of protons, and hence the same atomic number.
- Atoms of different elements have different atomic numbers.
Example
- Carbon: Atomic number Z=6. This means every carbon atom has 6 protons in its nucleus.
- Oxygen: Atomic number Z=8. This means every oxygen atom has 8 protons in its nucleus.
Mass Number (A)
In chemistry, the mass number, also known as the atomic mass number, is a key concept that represents the total number of protons and neutrons in the nucleus of an atom.
- It is denoted by the letter A .
- Protons and neutrons, collectively referred to as nucleons, are situated in the nucleus of an atom.
- For example, a carbon atom comprises 6 protons and 6 neutrons, resulting in a mass number of 12.
- While the number of protons remains constant for all atoms of a specific element, the number of neutrons can vary. Atoms of the same element with different mass numbers are known as isotopes.
- The mass of an electron is negligible in comparison to that of protons and neutrons, making its contribution to atomic mass minimal.
- Generally, the atomic mass of an atom is close to its mass number, although it can differ due to the presence of isotopes.
Representation of an Atom
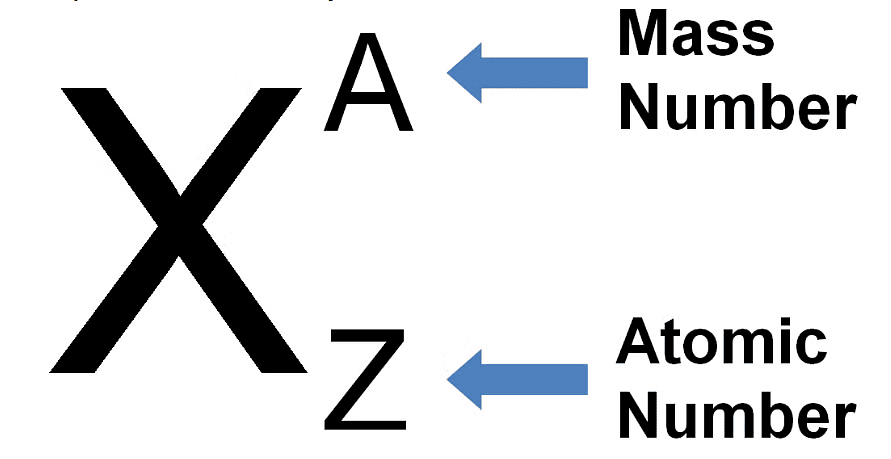 Representation of an Atom
Representation of an Atom
Example:
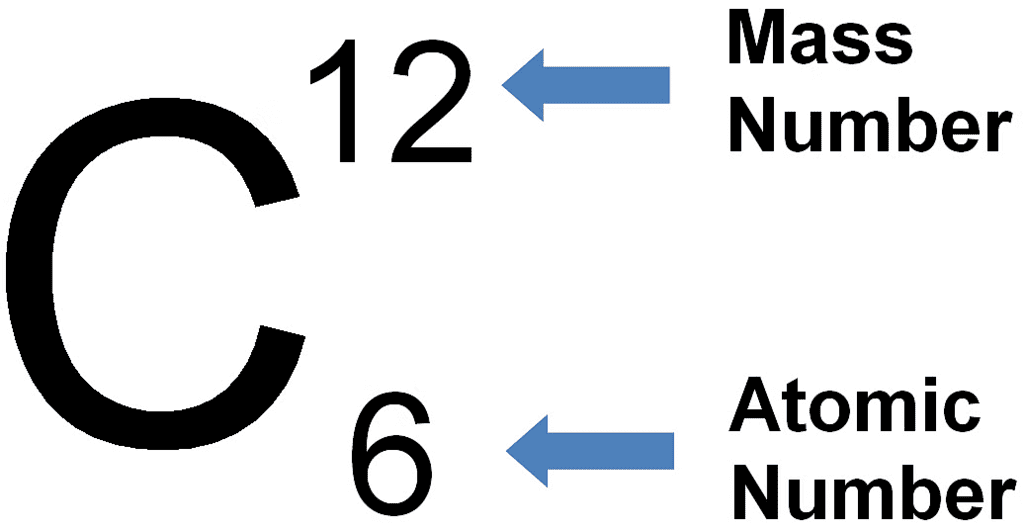 Example of Carbon Atom
Example of Carbon Atom
Difference Between Atomic Number and Mass Number
- The atomic number is important for understanding atoms. It tells us how many protons are in the nucleus of an atom. This number helps us know what the element is and where it is found in the periodic table.
- The mass number is different; it shows the total number of protons and neutrons in the nucleus. This gives a rough idea of how heavy the atom is.
- Both the atomic number and mass number relate to the nucleus of the atom, but they serve different purposes.
- The atomic number stays the same for every atom of a specific element, while the mass number can change. This happens because different versions of the same element, called isotopes, can have different numbers of neutrons.
- Knowing the difference between the atomic number and the mass number is important. It helps us study the structure of atoms, understand isotopes, and learn about how elements behave in the periodic table.
Different Types of Atomic Species
(i) Isotopes
Species with the same atomic number but a different mass number are called isotopes, e.g. 1H1, 1H2, 1H3.
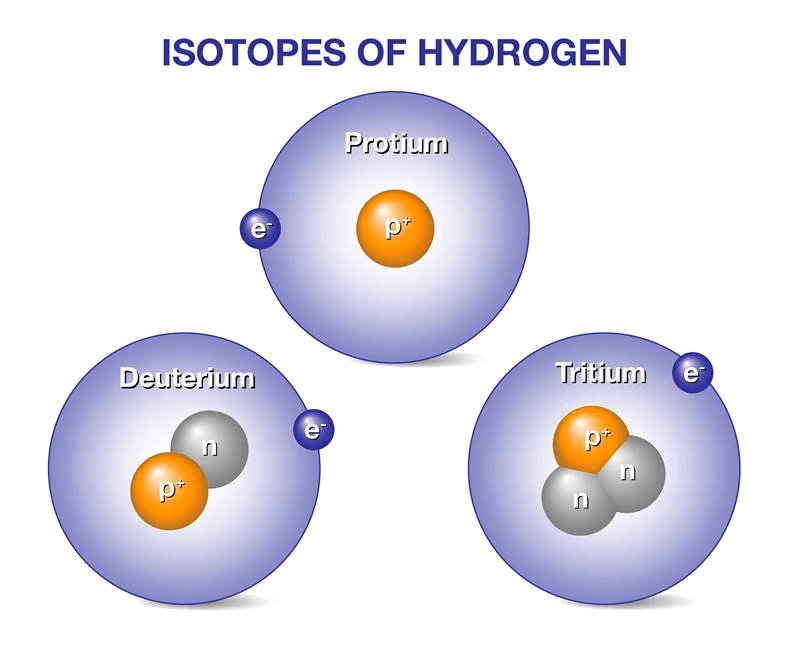 Isotopes of Hydrogen
Isotopes of Hydrogen
Uses of Isotopes:
- It is used as uranium for the nuclear reactor
- It is used as cobalt for treating blood cancer.
- Some isotopes are used in the process of labelling for various studies. Like, isotopes of hydrogen, carbon, nitrogen etc. are used for the labelling process.
- Used for nuclear imaging of various tissues for detection of diseases. fluorine-18, gallium-67, etc. are used for this purpose.
(ii) Isobars
Species with the same mass number but a different atomic number are called isobars, e.g. 18Ar40, 19K40, 20Ca40 all of them contain the same mass number 40 but different atomic number i.e 18, 19, and 20.
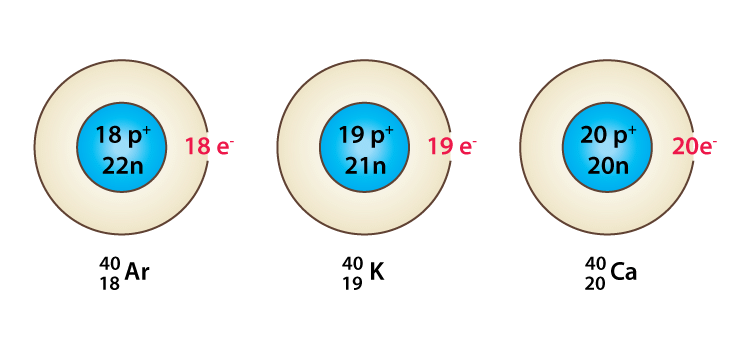 Example of Isobars
Example of Isobars
Differences between Isotopes and Isobars
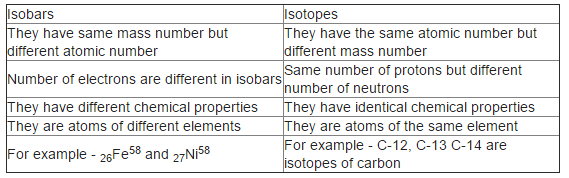 Differences between Isobars and Isotopes
Differences between Isobars and Isotopes
(iii) Isotones
Isotones are atoms or nuclei that have the same number of neutrons but different numbers of protons. This means that isotones belong to different elements but share a common neutron count.
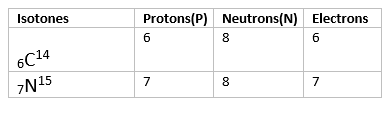
Key Points about Isotones:
- Same Neutron Number: Isotones have the same neutron number (N).
- Different Proton Number: Isotones have different atomic numbers (Z), meaning they are different elements.
- Example: Carbon-14 (14C6) and Nitrogen-15 (15N7) are isotones because both have 8 neutrons (14 - 6 = 8 and 15 - 7 = 8).
(iv) Isodiaphers
Species with the same isotopic number are called isodiaphers, e.g. 19K39, 19F9.
Isotopic number = mass number − × [2 atomic number]
(v) Isoelectronic
Isoelectronic species are atoms, ions, or molecules that have the same number of electrons. Despite having the same electron count, these species can have different atomic or molecular structures and compositions.
Key Points about Isoelectronic Species:
- Same Electron Number: Isoelectronic species have the same total number of electrons.
- Different Atomic or Molecular Identity: Isoelectronic species can be different elements, ions, or molecules.
Example: The fluoride ion (F -), the neon atom (Ne), and the sodium ion (Na+) are isoelectronic because each has 10 electrons.
Solved Questions
Q1: Isobars have the same number of ______?
(A) Protons
(B) Electrons
(C) Neutrons
(D) Nucleons
Solution: The answer is option 4 (Nucleons). The number of protons and neutrons alone will vary in isobars but the number of nucleons or the sum of protons and neutrons will always be the same.
Q2: Which of the following statements about Isobars is false?
(A) Their atomic numbers vary from each other.
(B) Chemically they are the same element but their forms are different.
(C) Physical properties can be similar to each other.
(D) They are different chemical elements having the same atomic mass.
Solution: Statement 2 is false. Isobars are different elements altogether.
Q3: If the atomic number of sodium is 11. Find out how many electrons and protons are present in a sodium atom.
Solution: We know that “Atomic Number = No. of Protons = No. of Electrons”
Thus, number of electrons=11 and number of protons =11
Q4: What is the atomic number of chlorine?
(A) 18
(B) 19
(C) 17
(D) 16
Solution: The correct answer is “C”. The atomic number of chlorine is 17 because the number of protons in a chlorine atom is 17.
Q5: Isotopes of a single element vary in the number of
(A) Neutrons
(B) Protons
(C) Electrons
(D) All of the above
Solution: The answer is "A" (Neutrons).
The number of protons will always constant in a single element. However, the number of neutrons can change. The number of neutrons varies in isotopes of an element but the number of protons always remains the same.
|
114 videos|263 docs|74 tests
|
FAQs on Atomic Number & Mass Number, Isotopes & Isobars - Chemistry Class 11 - NEET
| 1. What is the difference between atomic number and mass number? |  |
| 2. How is an atom represented in terms of its atomic number and mass number? |  |
| 3. What are the different types of atomic species based on their atomic number and mass number? |  |
| 4. Can two atoms have the same atomic number but different mass numbers? |  |
| 5. How are isotopes and isobars important in the study of atomic structure? |  |





















