Liquid State | Chemistry for JEE Main & Advanced PDF Download
| Table of contents |

|
| What is Liquid State? |

|
| 1. Vapor Pressure |

|
| 2. Surface Tension |

|
| 3. Viscosity |

|
What is Liquid State?
The liquid state is one of the primary phases of matter, characterized by specific properties that distinguish it from the solid and gaseous states.

Properties of liquids are stated below:
Definite Volume, Indefinite Shape: Liquids have a fixed volume but no fixed shape. They take the shape of their container.
Fluidity: Liquids can flow and easily change shape because the molecules are not fixed in place and can move past one another.
Intermolecular Forces: The molecules in a liquid are held together by intermolecular forces that are strong enough to keep the molecules close together but not strong enough to hold them in fixed positions as in a solid.
Density: Liquids are typically less dense than solids but more dense than gases. The molecules are closely packed but not in a rigid structure.
Incompressibility: Liquids are almost incompressible, meaning their volume doesn't change significantly with pressure.
Surface Tension: The surface of a liquid acts like a stretched elastic membrane due to the cohesive forces between molecules at the surface, leading to phenomena like droplets forming and insects walking on water.
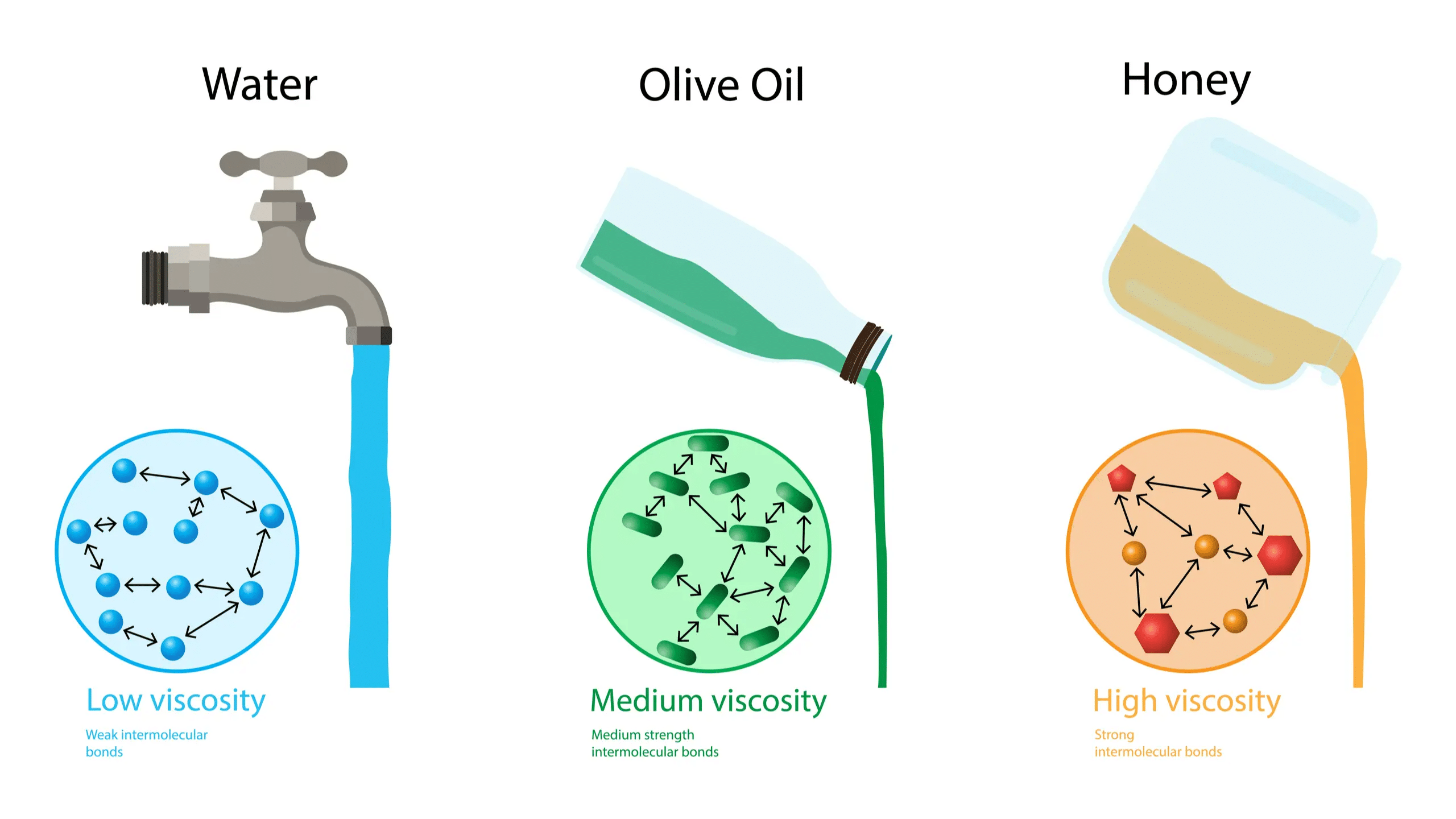
Viscosity: This is a measure of a liquid's resistance to flow. Higher viscosity means the liquid flows more slowly (e.g., honey), while lower viscosity means it flows more easily (e.g., water).
Diffusion: Molecules in a liquid can move and diffuse, though this process is slower compared to gases due to the closer proximity and intermolecular interactions of the molecules.
Temperature Effects: As temperature increases, the kinetic energy of the molecules increases, causing the viscosity to decrease and the liquid to eventually become a gas through evaporation or boiling.
Intermolecular forces are stronger in the liquid state than in the gaseous state. In liquids, molecules are very close together, with little empty space between them, making liquids denser than gases under normal conditions. These molecules are held together by attractive intermolecular forces, giving liquids a definite volume because the molecules do not separate from each other. However, molecules in liquids can move past one another freely, allowing liquids to flow, be poured, and take the shape of their container.
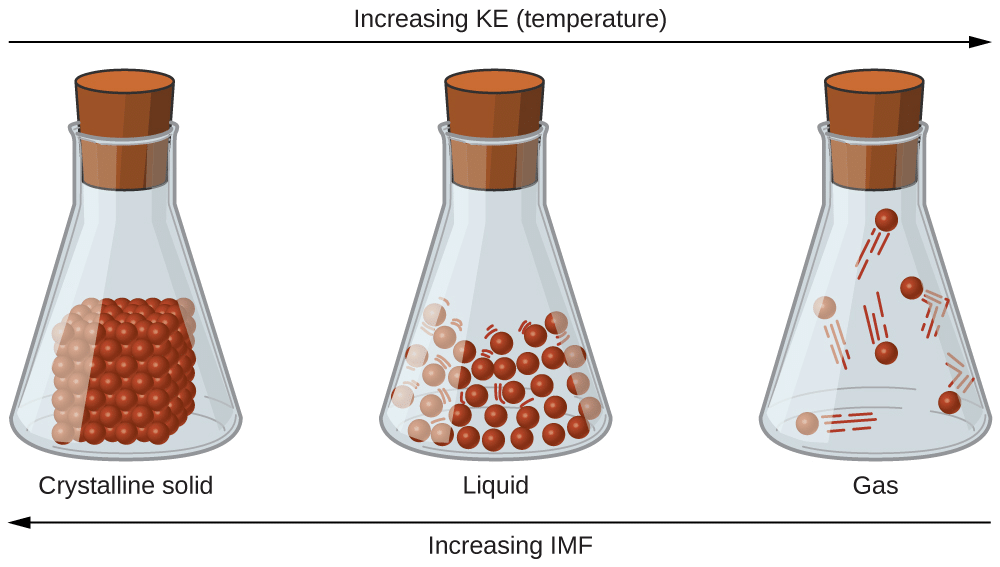 Increasing Intramolecular forces of attraction as we go from gases to solids
Increasing Intramolecular forces of attraction as we go from gases to solids
Let's look into some physical properties of liquids: vapor pressure, surface tension, and viscosity.
1. Vapor Pressure
If you partially fill an evacuated container with liquid, some of the liquid evaporates and fills the remaining space with vapor. Initially, the vapor pressure (pressure exerted by the vapor) increases, but after some time, it stabilizes when an equilibrium is established between the liquid and vapor phases. This constant pressure is called equilibrium vapor pressure or saturated vapor pressure, which depends on temperature.
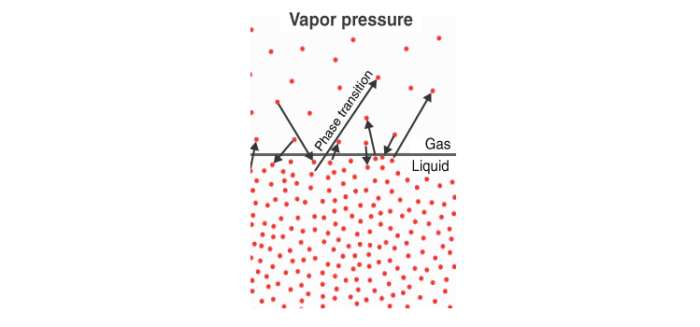
- When heating a liquid in an open vessel, it evaporates from the surface.
- Boiling occurs when the vapor pressure of the liquid equals the external pressure, allowing vaporization throughout the liquid.
- The boiling point is the temperature at which this happens. If the external pressure is 1 atm, it's called the normal boiling point. If the pressure is 1 bar, it's the standard boiling point, which is slightly lower than the normal boiling point because 1 bar is slightly less than 1 atm.
For example, water's normal boiling point is 100°C (373 K), and its standard boiling point is 99.6°C (372.6 K).
At high altitudes, where atmospheric pressure is lower, water boils at a lower temperature. Pressure cookers are used to cook food at high altitudes because they increase the pressure and thus the boiling point of water. In hospitals, autoclaves sterilize surgical instruments by increasing the pressure to raise the boiling point of water.
In a closed vessel, boiling doesn't occur because the vapor pressure keeps increasing with heat. Initially, there's a clear boundary between the dense liquid and less dense vapor. As temperature rises, the density of the vapor increases, and the liquid becomes less dense until they become equal, at which point the boundary disappears. This temperature is called the critical temperature.
2. Surface Tension
Liquids take the shape of their container, but small drops of mercury form spherical beads rather than spreading out. Soil particles at the bottom of a river remain separate, but stick together when taken out. Liquids rise or fall in a thin capillary tube when it touches the surface of the liquid. These phenomena are due to surface tension.
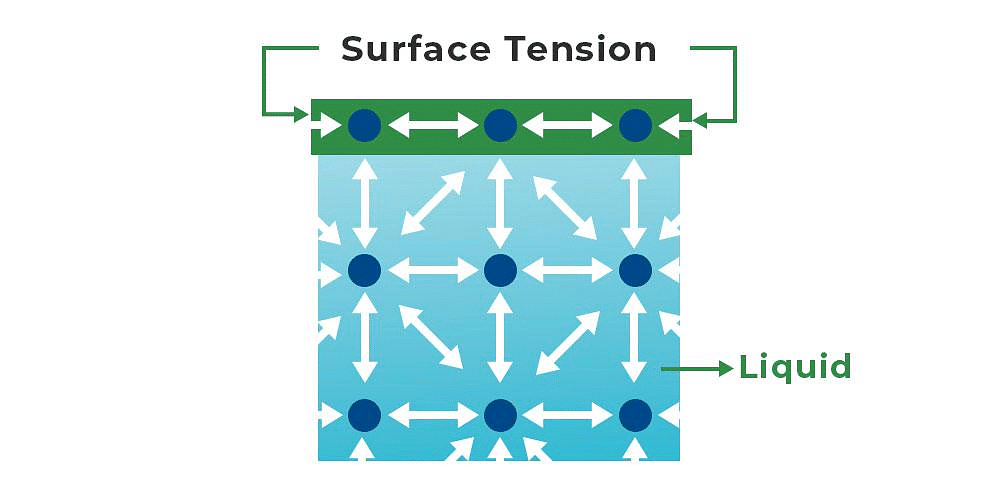
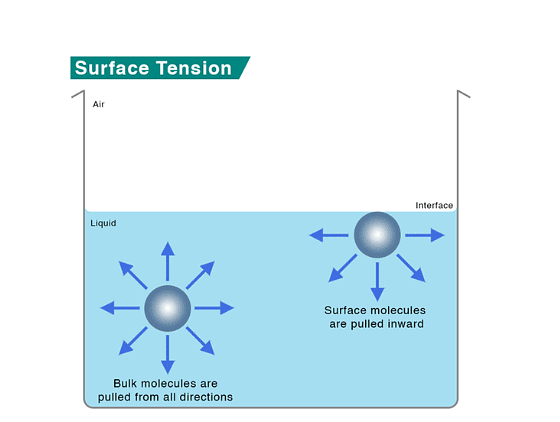
- A molecule in the bulk of a liquid experiences equal intermolecular forces from all sides, feeling no net force.
- However, a molecule on the surface feels a net attractive force towards the interior because there are no molecules above it.
- This makes the molecules at the surface have higher energy than those in the bulk. Liquids minimize their surface area because pulling a molecule from the bulk to the surface requires energy.
- The energy needed to increase the surface area by one unit is called surface energy.
Note:
Surface tension is the force acting per unit length on the surface of a liquid, denoted by the Greek letter γ (Gamma). It has the dimensions of kg·s⁻² and is measured in N·m⁻¹.
Liquids adopt a spherical shape to minimize surface area, which is why mercury drops are spherical. Heating sharp glass edges causes the glass to melt and form rounded shapes due to surface tension, a process known as fire polishing.
Liquids rise or fall in a capillary tube because of surface tension. Liquids wet surfaces by spreading out as a thin film. Moist soil grains stick together because the surface area of the water film is minimized. Surface tension gives the liquid surface its stretching property, and droplets are slightly flattened by gravity, though in a gravity-free environment, they would be perfectly spherical.
The magnitude of surface tension depends on the intermolecular forces in the liquid. Stronger attractive forces result in higher surface tension. Increasing the temperature raises the kinetic energy of the molecules, reducing the effectiveness of intermolecular attractions, and thus decreasing surface tension.
3. Viscosity
Viscosity is a key property of liquids that measures how much they resist flowing. This resistance is due to internal friction between layers of the liquid as they move past each other. Strong intermolecular forces between molecules make it harder for these layers to move.
When a liquid flows over a fixed surface, the layer of molecules touching the surface stays still. The velocity of the liquid increases with distance from this fixed layer, creating a smooth flow called laminar flow. In laminar flow, each layer influences the flow of its neighboring layers: the layer above speeds it up, and the layer below slows it down.
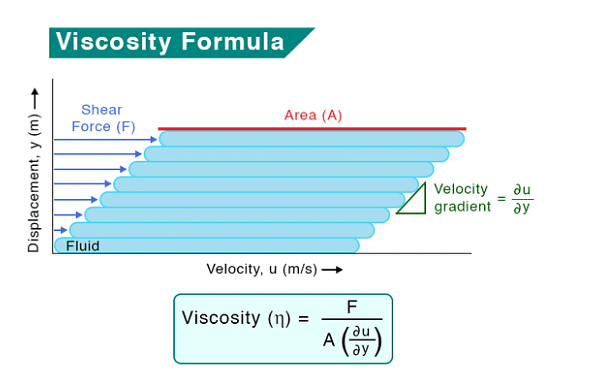
If the velocity of the layer at a distance dz is changed by a value du then velocity gradient is given by the amount 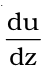
A force is required to maintain the flow of layers. This force is proportional to the area of contact of layers and velocity gradient i.e.
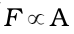
and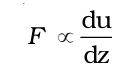
Therefore,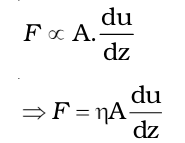
where.
- A is the area of contact
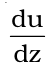 is the velocity gradient; the change in velocity with distance
is the velocity gradient; the change in velocity with distance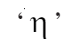 is proportionality constant and is called coefficient of viscosity
is proportionality constant and is called coefficient of viscosity
Here, (the coefficient of viscosity) is the proportionality constant. It represents the force required when the velocity gradient is one unit and the area of contact is one square meter.
Note:
The SI unit for viscosity is the Pascal - second (Pa·s), and in the cgs system, it's called the poise.
Liquids with higher viscosity flow more slowly. Strong forces like hydrogen bonds and van der Waals forces increase viscosity. Glass is an example of an extremely viscous liquid, appearing solid but actually flowing very slowly over time, as seen in old windowpanes that are thicker at the bottom.
Viscosity decreases with rising temperature because molecules move faster and can overcome intermolecular forces more easily, allowing the liquid to flow more freely.
|
334 videos|651 docs|300 tests
|
FAQs on Liquid State - Chemistry for JEE Main & Advanced
| 1. What is the relationship between temperature and vapor pressure in a liquid state? |  |
| 2. How does surface tension affect the behavior of liquids in a liquid state? |  |
| 3. What is viscosity, and how does it impact the flow of liquids in a liquid state? |  |
| 4. How does the intermolecular forces between molecules affect the properties of liquids in a liquid state? |  |
| 5. How can we measure the vapor pressure of a liquid in a liquid state experimentally? |  |















