Biological Importance of Na, K, Mg & Ca | Inorganic Chemistry for NEET PDF Download
Biological Importance or Significance of Magnesium
- Magnesium is essential for the activity of the various enzymes such as enzymes of glycolysis.
- It is the central atom present in chlorophyll (plant pigment necessary for photosynthesis).
- It is a cofactor for the breakdown of the fats and glucose.
- It is essential for the synthesis of the energy currency of the cell, that is, ATP.
- Responsible for the stability and synthesis of DNA.
- Maintains the electrolyte balance in the body.
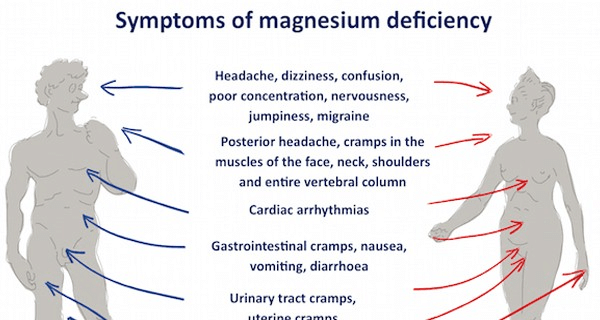
- Magnesium deficiency is associated with sleep disorder.
- Deficiency also leads to abnormal heart rhythms.
Uses of Magnesium
Magnesium alloys such as duralumin and magnalium are used in making flares, fuse for thermite.
- In manufacturing of engine parts and wheels.
- Preparation of malleable cast iron.
- Used to remove Sulphur during production of iron and steel.
- As a reducing agent to separate uranium from the mixture of other elements.
- It is also needed for blood glucose control.
Biological Importance of Calcium
- Important component of cell wall. It is present in the middle lamellae in the form of calcium pectate.
- Maintains the anionic balance in the plant vacuole.
- Used to stabilize the permeability of cell membranes.
- Important for structure and function of proteins.
- Essential component during blood clotting.
- It also brings about muscle contraction.
- Calcium acts as secondary messenger during cell signaling.
- Helps in proper heart and nerve functions.
- Calcium is essential for strong bones and teeth.
Calcium- Magnesium Balance
The calcium and magnesium are so important that they are known as Twin Minerals. The ideal ratio of calcium and magnesium is 1:1. Both have opposite effects. For Example, if calcium contracts muscle, magnesium relaxes muscle. Low magnesium intake will increase more storage of calcium in the body.
Imbalance in magnesium allows too much calcium to be stored in the body.
Role of Magnesium in Calcium Absorption
Magnesium is essential for calcium absorption. Without magnesium, calcium may get deposited in soft tissues and can cause arthritis. Without magnesium, calcium cannot be reabsorbed.
Frequently Questions(FAQs)
Q1. Is magnesium water soluble in the body?
Sol. Yes, magnesium is water soluble in the body.
Q2. What is meant by Mg and Mg2+?
Sol. Mg represents the symbol for magnesium or magnesium atom and Mg2+ represents magnesium ions formed after losing two valence electrons.
Organic Uses Of Sodium And Potassium: Sodium Potassium Pump
It is observed that the content of sodium and potassium in a human body is more than iron and copper which tend to get the main focus in a human diet. These biologically important compounds perform a function of a power generator under the roof of a cell. They act as communicating agents to transmit information such as flexing of muscles etc. For an instant, if you want to jump, these transmitters carry the information to the brain which gives the command to jump. Neurons used these ions for communicating the required information. The concentration of the ions varies on either side of the cell membrane. In the plasma of the blood, potassium content is 5 mmolL-1 and sodium content is 143 mmol L-1.
Use of Sodium
Sodium ions are usually found at the surface of the cells in the blood plasma and interstitial fluids which acts as surrounding to the cell. These are mainly used to transmit information, in the transportation of the sugar and amino acids within the cell etc.
Use of Potassium
Potassium ions are a cation and they are present in the cell fluids. It is used to activate enzymes, oxidation of sugars and also responsible for transmitting the information.
Role of Sodium And Potassium in conduction
Water in its pure form does not possess the capability to conduct electricity. Sodium and potassium ions along with other electrolytes present in the body are responsible for the conduction of electricity through water. These ions move to and fro from the cell. Every time they move they conduct electricity. Proper electrolyte balance plays a vital role in the human body.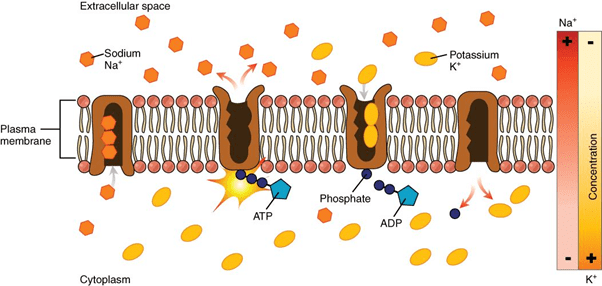 Sodium-Potassium Pump
Sodium-Potassium Pump
High consumption of sodium and low intake of potassium results in higher risk of death and heart diseases. Making the right choice in your food diet plays a very important role for a healthy body.
|
80 videos|112 docs|110 tests
|
FAQs on Biological Importance of Na, K, Mg & Ca - Inorganic Chemistry for NEET
| 1. What is the biological importance of sodium (Na)? |  |
| 2. How does potassium (K) contribute to biological functions? |  |
| 3. What is the significance of magnesium (Mg) in biological systems? |  |
| 4. How does calcium (Ca) contribute to biological functions? |  |
| 5. What happens if there is an imbalance in the levels of these minerals in the body? |  |
















