Lakhmir Singh & Manjit Kaur: Metals and Non-metals, Solutions- 1 | Science Class 10 PDF Download
(Page No : 131)
Question 1:
Name one metal and one non-metal which exist in liquid state at room temperature.
Solution :
Metal – Mercury.
Non metal – Bromine.
Question 2:
Why are metals called electropositive elements whereas non-metals are called electronegative elements ?
Solution :
Metals are electropositive elements because they can form positive ions by losing electrons.
Non-metals are electronegative elements because they can form negative ions by gaining electrons.
Question 3:
(a) Name the most abundant metal in the earth’s crust.
(b) Name the most abundant non-metal in the earth’s crust.
Solution :
(a) Aluminium.
(b) Oxygen.
(Page No: 132)
Question 4:
Name one metal that has a low melting point.
Solution :
Cesium.
Question 5:
Name the metal which is the poorest conductor of heat.
Solution :
Lead.
Question 6:
State whether the following statement is true or false :
Non-metals react with dilute acids to produce a gas that burns with a ‘pop’ sound.
Solution :
False.
Question 7:
From amongst the metals sodium, calcium, aluminium, copper and magnesium, name the metal :
(i) which reacts with water only on boiling, and
(ii) another which does not react even with steam.
Solution :
(i) Aluminium.
(ii) Copper.
Question 8:
What changes in the color of iron nails and copper sulphate solution do you observe after keeping the iron nails dipped in copper sulphate solution for about 30 minutes?
Solution :
The iron nail gets covered with a red-brown coating of copper metal; The blue colour of copper sulphate solution fades gradually.
Question 9:
What is aqua-regia? Name two special metals which are insoluble in common reagents but dissolve in aqua-regia.
Solution :
Aqua-regia is a freshly prepared mixture of one part of concentrated nitric acid and 3 parts of concentrated hydrochloric acid. Gold and platinum dissolve in aqua-regia
Question 10:
Give the names and formulae of (a) two acidic oxides, and (b) two basic oxides.
Solution :
(a) Carbon dioxide and sulphur dioxide.
(b) Sodium oxide and magnesium oxide.
Question 11:
What name is given to those metal oxides which show basic as well as acidic behaviour ?
Solution :
Amphoteric oxides.
Question 12:
Name two metals which form amphoteric oxides.
Solution :
Aluminium and zinc.
Question 13:
A copper coin is kept immersed in a solution of silver nitrate for some time. What will happen to the coin and the colour of the solution ?
Solution :
Copper coin will get a shining greyish white coating of silver metal. The color of the solution will turn blue.
Question 14:
Which property of copper and aluminium makes them suitable :
(a) for making cooking utensils and boilers ?
(b) for making electric wires ?
Solution :
(a) High thermal conductivity.
(b) High electrical conductivity.
Question 15:
Write the names and formulae of (a) a metal hydride, and (b) a non-metal hydride.
Solution :
Sodium hydride,Hydrogen sulphide
Question 16:
Name the metal which has been placed :
(a) at the bottom of the reactivity series
(b) at the top of the reactivity series
(c) just below copper in the reactivity series
Solution :
(a) Gold.
(b) Potassium.
(c) Mercury.
Question 17:
Which of the two metals is more reactive : copper or silver ?
Solution :
Copper.
Question 18:
(a) Name one metal which is stored in kerosene oil.
(b) Name one non-metal which is stored under water.
Solution :
(a) Sodium.
(b) White phosphorus.
Question 19:
Write an equation for the reaction of :
(a) sodium with oxygen
(b) magnesium with oxygen
Solution :
(a) 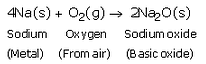
(b)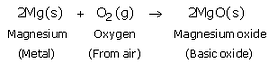
Question 20:
Name two metals which are used :
(a) for making electric wires.
(b) for making domestic utensils and factory equipment.
(c) for making jewelry and decorating sweets.
Solution :
(a) Aluminium and copper.
(b) Copper and aluminium.
(c) Gold and silver.
Question 21:
Which metal foil is used for packing some of the medicine tablets ?
Solution :
Aluminium foil.
Question 22:
Name the non-metal which is used :
(a) to convert vegetable oil into vegetable ghee(solid fat).
(b) as a rocket fuel (in liquid form).
(c) to make electrodes of dry cells.
(d) to preserve food materials.
(e) in the vulcanization of rubber.
Solution :
(a) Hydrogen.
(b) Hydrogen.
(c) Carbon.
(d) Nitrogen.
(e) Sulphur.
Question 23:
Name one property which is characteristic of (a) metals, and (b) non-metals.
Solution :
(a) Metals are malleable.
(b) Non-metals are non-malleable.
Question 24:
What is meant by “brittleness” ? Which type of elements usually show brittleness : metals or non-metals ?
Solution :
Brittleness is the property of being brittle i.e. breaking easily.
Non-metals show brittleness.
Question 25:
What will happen if a strip of zinc is immersed in a solution of copper sulphate ?
Solution :
When a strip of zinc metal is put in copper sulphate solution, then the blue colour of copper sulphate solution fades gradually and red brown coating of copper is deposited on zinc strip.
Question 26:
What will happen if a strip of copper is kept immersed in a solution of silver nitrate (AgN03) ?
Solution :
When a strip of copper metal is immersed in silver nitrate solution, the solution gradually becomes blue and a shining greyish-white deposit of silver metal is formed on copper strip.
Question 27:
What happens when iron nails are put into copper sulphate solution ?
Solution :
When iron nails are placed in copper sulphate solution, the blue colour of copper sulphate solution fades gradually and red-brown copper metal is formed.
Question 28:
How would you show that silver is chemically less reactive than copper ?
Solution :
If a strip of silver metal is kept immersed in copper sulphate solution for some time, then no reaction occurs. This shows that silver is not able to displace copper from copper sulphate solution.
Question 29:
Give reasons for the following :
Blue colour of copper sulphate solution is destroyed when iron filings are added to it.
Solution :
Blue color of copper sulphate is destroyed because iron displaces copper from copper sulphate solution as iron is more reactive than copper.
|
85 videos|437 docs|75 tests
|
FAQs on Lakhmir Singh & Manjit Kaur: Metals and Non-metals, Solutions- 1 - Science Class 10
| 1. What are metals and non-metals? |  |
| 2. What are some examples of metals and non-metals? |  |
| 3. How are metals and non-metals different in terms of their physical properties? |  |
| 4. What are some common uses of metals and non-metals? |  |
| 5. How do metals and non-metals react with other elements? |  |

|
Explore Courses for Class 10 exam
|

|

















