Integer Answer Type Questions: Structure of Atom | JEE Advanced | 35 Years Chapter wise Previous Year Solved Papers for JEE PDF Download
Q.1. The work function φ of some metals is listed below. The number of metals which will show photoelectric effect when light of 300 nm wavelength falls on the metal is (2011)
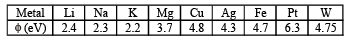
Ans. Sol. (4)
Energy associated with incident photon =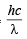
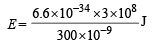
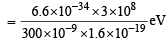 = 4.16 eV
= 4.16 eV
Photoelectric effect can take place only when Ephoton > φ
Thus, number of metals showing photoelectric effect will be 4 (i.e. Li, Na, K and Mg).
Q.2. The maximum number of electrons that can have principal quantum number, n = 3, and spin quantum ms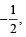 is (2011)
is (2011)
Ans. Sol. (9)
Maximum number of electrons (n2) when n = 3 = 32 = 9
∴ Number of orbitals = 9
∴ Number of electrons with ms =-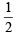 will be 9.
will be 9.
Q.3. The atomic masses of ‘He’ and ‘Ne’ are 4 and 20 a.m.u., respectively. The value of the de Broglie wavelength of ‘He’ gas at —73°C is “M” times that of the de Broglie wavelength of ‘Ne’ at 727°C ‘M’ is (JEE Adv. 2013)
Ans. Sol. (5) Since,
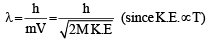
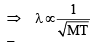
For two gases,
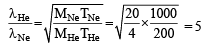
Q.4. In an atom, the total number of electrons having quantum numbers n = 4, |m1| = 1 and ms = -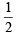 is (JEE Adv. 2014)
is (JEE Adv. 2014)
Ans. Sol. (6) |mi| =1 means ml can be +1 and –1.
So, for n = 4, six orbitals are possible and each has 1 electron with s = -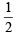 . So total number of electrons = 6.
. So total number of electrons = 6.
Q.5. Not considering the electronic spin, the degeneracy of the second excited state (n = 3) of H atom is 9, while the degeneracy of the second excited state of H– is (JEE Adv. 2015)
Ans. Sol. (3) Ground state configuration:

in second excited state, electron will jump from 1s to 2p, so degeneracy of second excited state of H– is 3.
|
347 docs|185 tests
|
FAQs on Integer Answer Type Questions: Structure of Atom - JEE Advanced - 35 Years Chapter wise Previous Year Solved Papers for JEE
| 1. What is the structure of an atom? |  |
| 2. How are protons, neutrons, and electrons arranged in an atom? |  |
| 3. What are the properties of protons, neutrons, and electrons? |  |
| 4. How does the number of protons, neutrons, and electrons determine the identity of an atom? |  |
| 5. What is the significance of the energy levels or shells in an atom? |  |
|
347 docs|185 tests
|

|
Explore Courses for JEE exam
|

|

















