JEE Advanced (Fill in the Blanks): Organic Chemistry - Some Basic Principles & Technique | Chapter-wise Tests for JEE Main & Advanced PDF Download
Fill in the Blanks
Q.1. Among the given cations, ................. is most stable. (1981)
(sec-butyl carbonium ion; tert-butyl carbonium ion; n-butyl carbonium ion)
Ans. tert-butyl carbonium ion
Solution. tert-butyl carbonium ion is more stable due to liyperconjugation and +1 effect of methyl groups.
Q.2. The compound having both sp and sp2 hybridized carbon atoms is ................. . (1981)
(propene, propane, propadiene)
Ans. propadiene
Q.3. ................. ring is most strained. (1981)
(Cyclopropane, Cyclobutane, Cyclopentane)
Ans. cyclopropane
Solution. cyclopropane, because it has maximum deviation, from the normal bond angle of 109°28' present in alkanes. In it bond angle is 60°.
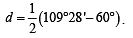
Q.4. The terminal carbon atom in butane is ............... hybridised. (1985)
Ans. sp3
Solution. sp3
Q.5. A ............... diol has two hydroxyl groups on ............... carbon atoms. (1986)
Ans. vicinal, adjacent
Solution. vicinal, adjacent (or stable, different).
Q.6. Isomers which are ............... mirror images are known as ............... . (1988)
(superimposable, non-superimposable, enantiomers, diastereomers, epimers)
Ans. non-superimposable, enantiomers
Solution. non-superimposable, enantiomers;
Q.7. The valence atomic orbitals on carbon in silver acetylide is ............... hybridized. (1990)
Ans. sp
Solution. sp;
Q.8. The kind of delocalization involving sigma bond orbitals is called ............... . (1994)
Ans. hyperconjugation
Solution. Hyperconjugation;
Q.9. The IUPAC name of succinic acid is ............... . (1994)
Ans. butane–1, 4-dioic acid
Solution. Butane-1, 4-dioic acid; Succinic acid has the formula.
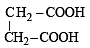
True/False
Q.1. Iodide is a better nucleophile than bromide. (1985 - ½ Mark)
Ans. F
Solution. Iodide is bigger in size than bromide, hence its electrons are more dispersed than that of bromide, with the result it is weaker nucleophile than bromide.
Q.2. An electron donating substituent in benzene orients the incoming electrophilic group to the meta position. (1987)
Ans. F
Solution. An electron-donating group increases the electron density in o- and p- positions due to +M, +E and/or +I effects and hence orients the new electrophile to o- and p- positions.
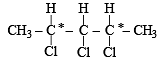
Q.3. 2, 3, 4-Trichloropentane has three asymmetric carbon atoms. (1990)
Ans. F
Solution. There are only two asymmetric (marked with *) carbon atoms.
Q.4. During SN1 reaction, the leaving group leaves the molecule before the incoming group is attached to the molecule.
Ans. T
Solution. In SN1 (un imolecular n ucleophilic substitution reaction), the leaving group leaves, thus producing a carbocation followed by the addition of the incoming group.
|
446 docs|930 tests
|

|
Explore Courses for JEE exam
|

|

















