Cope & Claisen Rearrangement Reactions | Organic Chemistry PDF Download
Cope Rearrangement
The thermal [3,3]-sigmatropic rearrangement of 1,5-dienes to the isomeric 1,5dienes is called the Cope
rearrangement, and it can only be detected when the 1,5-diene substrate is substituted.
- The [3,3] sigmatropic rearrangement of acyclic hexa-1,5-dienes typically takes place around 250-300 oC and the activation energy is determined to be 35 kcal / mole.
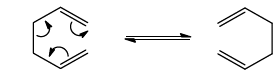
- Substituents that can be brought into conjugation during the rearrangement favours it in one direction and lowers the activation energy of such a process.
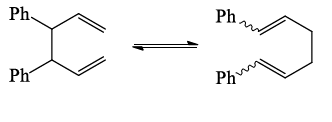
Oxy-Cope rearrangement
- Introduction of alkoxy substitution (oxy-Cope system)
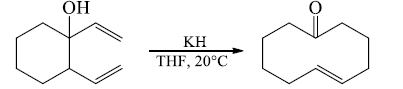
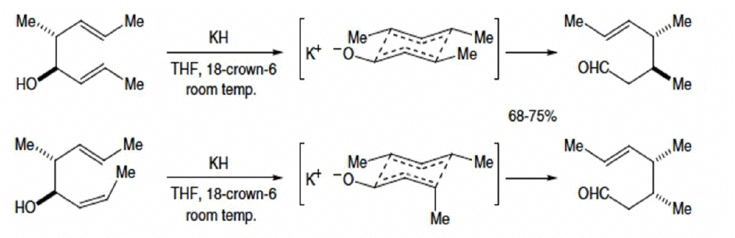
Stereochemical outcome of oxy-Cope
- Prefers the chair-shaped transition state
- The ratio of stereoisomers of the product depends on the orientation of the substituents in the transition state
- A substituent at C-3 (or C-4) of the 1, 5-diene generally prefers the less-hindered equatorial position and this leads to the E alkene isomer of the product.
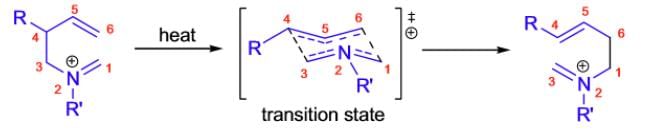
Aza-Cope rearrangement
- Mild conditions, but reversible, so not synthetically as useful.
- With R=OH, the reaction can be coupled with intramolecular Mannich reaction, to provide substituted pyrrolidines.
When 1, 5-dienes are heated, they isomerize via a [3,3]-sigmatropic rearrangement known as the Coperearrangement. The rearrangement of N-substituted 1, 5-dienes is called the aza-Cope rearrangement.
Mechanism:
The aza-Cope rearrangement is a concerted process, and it usually takes place via a chair like transition state where the substituents are arranged in a quasi-equatorial position. (See more detail in Cope rearrangement.)
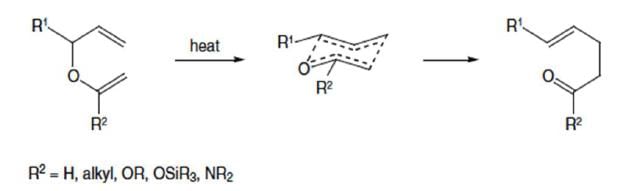
Claisen Rearrangement
The thermal [3, 3]-sigmatropic rearrangement of allyl vinyl ethers to the corresponding γ,δ-unsaturated carbonyl compounds is called the Claisen rearrangement
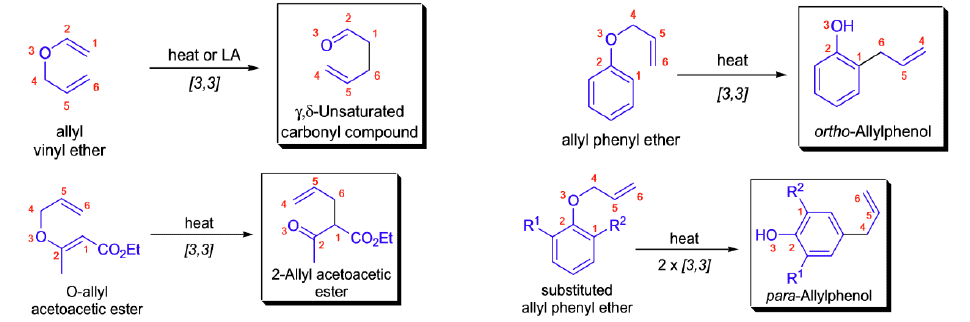
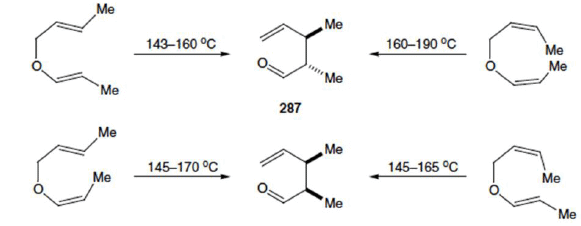
Stereochemical Aspects
The relative stereochemistry across the new carbon-carbon single bond is established as a result of the chair-like transition state and depends on the geometry of the double bonds in the starting material. e.g.
Aromatic Claisen Rearrangement of allyl phenyl ether
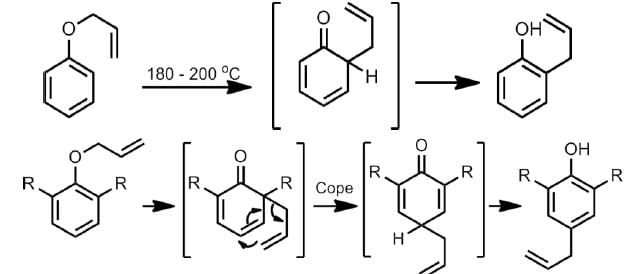
Aza-Claisen Rearrangement
The thermal [3,3]-sigmatropic rearrangement of allyl vinyl ethers is called the Claisen rearrangement. Its variant, the thermal [3,3]-sigmatropic rearrangement of N-allyl enamines, is called the aza-Claisen rearrangement
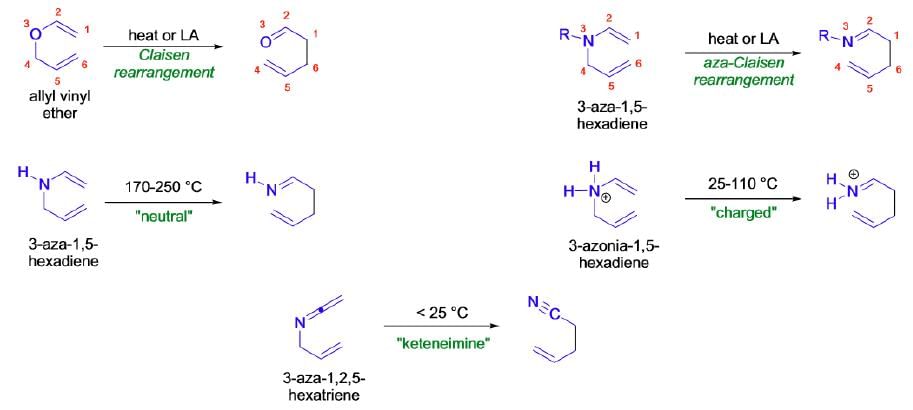
Mechanism:
The Aza-Claisen rearrangement is a concerted process, and it usually takes place via a chair like transition state where the substituents are arranged in quasi-equatorial positions. (See more details in Claisen rearrangement.)
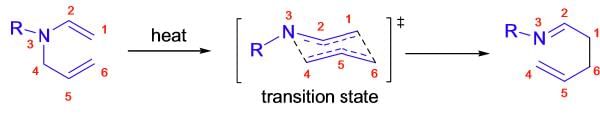
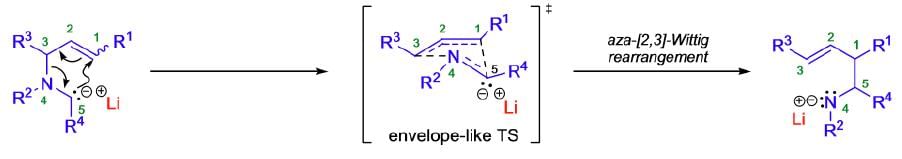
Aza-Wittig Rearrangement
It involves the isomerization of α-metalated tertiary amines to skeletally rearranged metal amides.
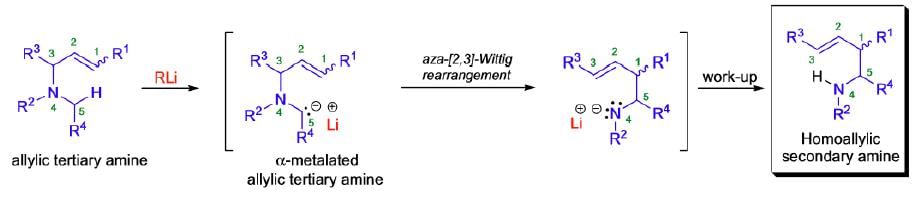
Mechanism:
The aza-[2,3]-Wittig rearrangement proceeds by a concerted process through a six-electron, five-membered cyclic transition state of envelope-like geometry. According to the Woodward-Hoffmann rules, the [2,3]-sigmatropic rearrangement is a thermally allowed, concerted sigmatropic rearrangement that proceeds in a suprafacial fashion with respect to both fragments. Therefore, the aza-[2,3]-Wittig rearrangement is a one-step SN1-reaction, which results in a regiospecific carbon-carbon bond formation by suprafacial allyl inversion in which the heteroatom function gets transposed from allylic to homoallylic. The driving force for these rearrangements is the transfer of a formal negative charge from the less electronegative α-carbon to the more electronegative heteroatom.
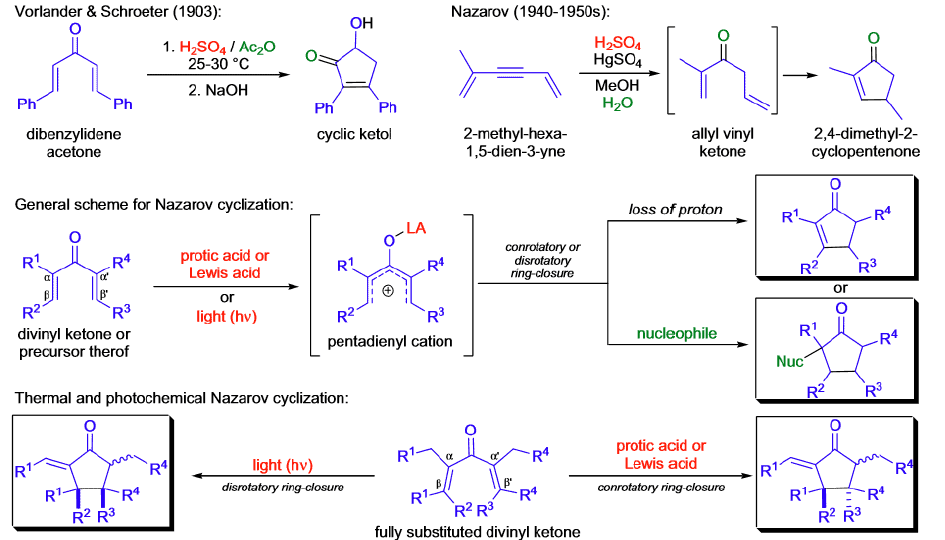
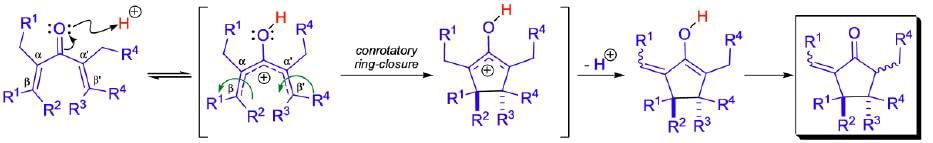
Nazarov Cyclization
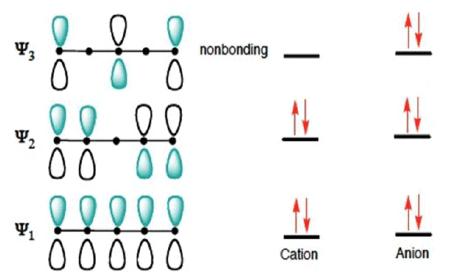
The cyclization proceeds through conrotation. Use of FMO explains this outcome. Here, Ψ2 is HOMO.
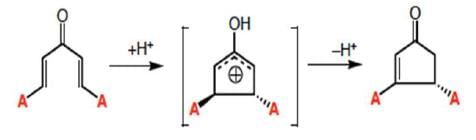
The Nazarov cyclization is a pericyclic reaction that belongs to the class of 4π electrocyclizations. The first step is the coordination of the Lewis acid to the carbonyl group of the substrate and the formation of the pentadienylic cation, which undergoes a conrotatory ring closure to give a cyclic carbocation that may be captured by a nucleophile, may undergo deprotonation, or further rearrangement may take place. The electrocyclization step may proceed in a clockwise or counterclockwise fashion (torquoselectivity) generating two diastereomers when the divinyl ketone substrate is chiral.
Mechanism:
Sommelet-Hauser Rearrangement
The [2,3]-sigmatropic rearrangement of benzylic quaternary ammonium salts in the presence of a strong base is known as the Sommelet Hauser rearrangement (S.-H. rearrangement).
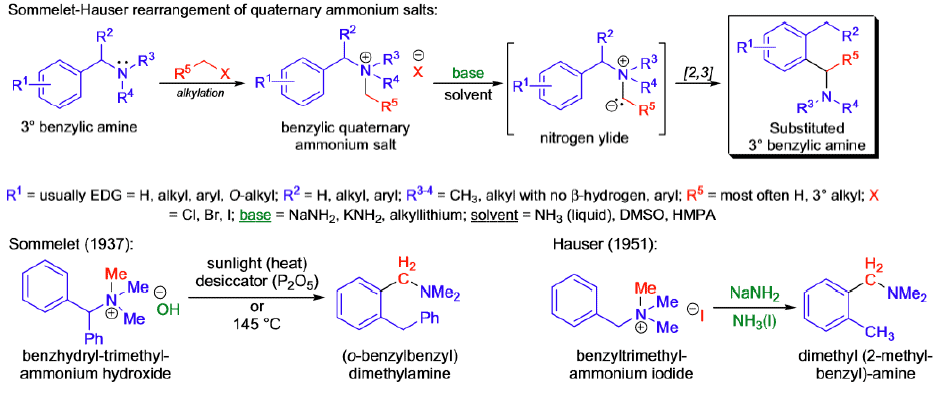
Mechanism:
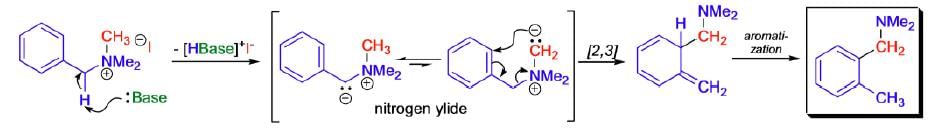
|
44 videos|102 docs|52 tests
|
FAQs on Cope & Claisen Rearrangement Reactions - Organic Chemistry
| 1. What is the Cope rearrangement reaction? |  |
| 2. What is the Claisen rearrangement reaction? |  |
| 3. What are the key differences between the Cope and Claisen rearrangement reactions? |  |
| 4. What are the applications of the Cope and Claisen rearrangement reactions? |  |
| 5. Are there any limitations or challenges associated with the Cope and Claisen rearrangement reactions? |  |

















