Introduction to Biomolecules & Carbohydrates | Organic Chemistry PDF Download
Introduction to Bio-Molecules
Biomolecules are the most essential organic molecules, which are involved in the maintenance and metabolic processes of living organisms.
- These non-living molecules are the actual foot-soldiers of the battle of sustenance of life.
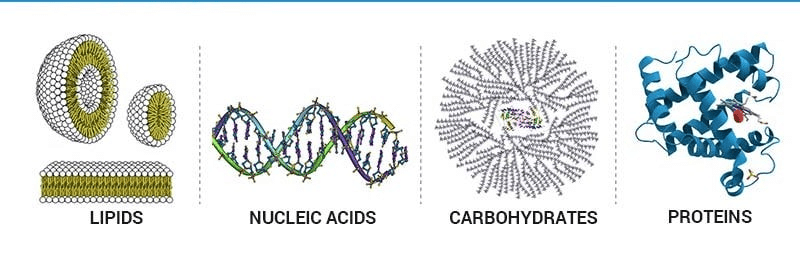 Different Classes of Carbohydrates
Different Classes of Carbohydrates
- They range from small molecules such as primary and secondary metabolites and hormones to large macromolecules like proteins, nucleic acids, carbohydrates, lipids, etc.
- These are certain complex organic molecules that form the basis of life. These build up living organisms and are also required for their growth and maintenance.
- The main classes of biomolecules are
1. Carbohydrates
2. Nucleic acids
3. Amino Acids
4. Peptides
5. Terpenes
6. Lipids
7. Alkaloids
Carbohydrates
Carbohydrates are macronutrients and are one of the three main ways by which our body obtains its energy. The carbohydrates may be defined as polyhydroxy aldehydes or ketones or substances which give such molecules on hydrolysis.

- Carbohydrates form a very large group of naturally occurring organic compounds which play a vital role in daily life.
- They are produced in plants by the process of photosynthesis. After eating plant foods, humans convert carbohydrates into glucose.
- The most common carbohydrates are glucose, fructose, sucrose, starch, cellulose etc.
- Many carbohydrates are sweet in taste and all sweet carbohydrates are called as sugars. The chemical name of the most commonly used sugar in our homes is sucrose.
Functions of Carbohydrate
1. Provide energy
2. Maintain blood glucose
3. Spare protein
4. Prevents ketosis
Classification of Carbohydrates
A. Depending upon their behavior on Hydrolysis
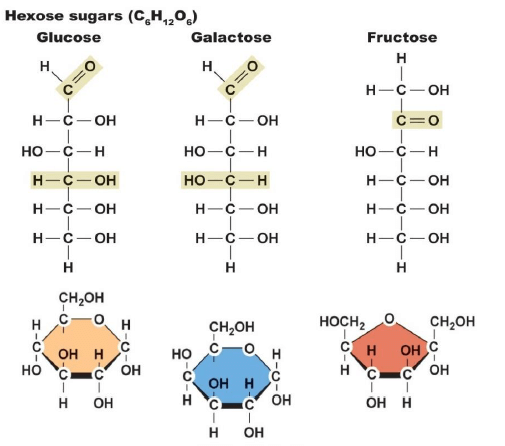
(1) Monosaccharides:
- Polyhydroxy aldehyde or ketone which cannot be hydrolyzed further to a smaller molecule containing these functional groups, is known as a monosaccharide. About 20 monosaccharides occur in nature and glucose is the most common amongst them.
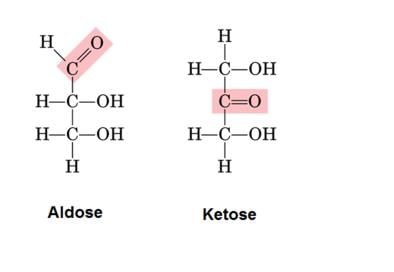
- Monosaccharides are further classified on the basis of the functional group present in them. If a monosaccharide contains an aldehyde group, it is known as an aldose and if it contains a keto group, it is known as a ketose.
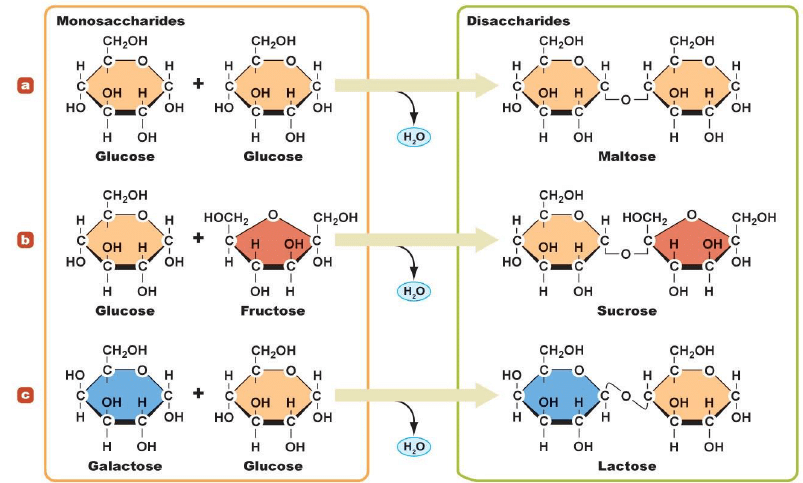
(2) Disaccharides:
Carbohydrates that give two monosaccharide molecules on hydrolysis are called disaccharides e.g. sucrose, maltose, lactose, etc.
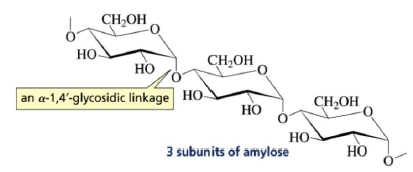
(3) Polysaccharides:
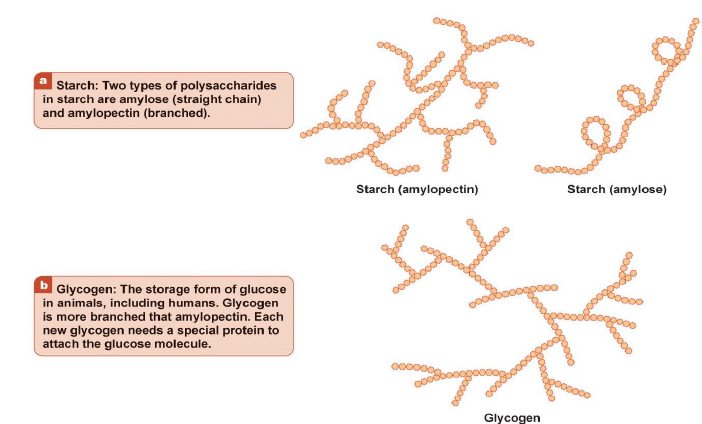
Carbohydrates yield a large number of monosaccharide units on hydrolysis e.g. starch, glycogen, cellulose etc.
Amylose: It is a component of starch.
Amylopectin: It is another polysaccharide component of starch that has a branched structure.
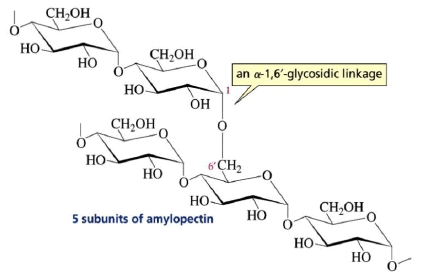
The Comparison of Starch and Glycogen Molecules
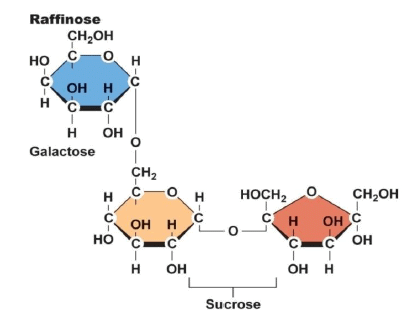
(4). Oligosaccharides:
- Similar in length to simple carbohydrates and in makeup to polysaccharides. Humans lack the enzymes necessary to digest them.
- Intestinal microflora digest and ferment them and cause bloating, discomfort, and flatulence. Food sources are Legumes, beans, cabbage, brussels sprouts, broccoli.
The Structure of an Oligosaccharide
B. Depending upon Functional Group
1. Aldoses: They are aldehyde-containing carbohydrates. At C1, the carbonyl is an aldehyde.
2. Ketoses: They are ketone-containing carbohydrates. At any other carbon, carbonyl present would be the ketonic group.
C. Depending upon no. of Carbon Atoms
(a) Aldoses
(i) Three carbons: Aldotriose
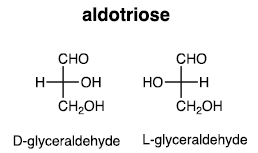
(ii) Four carbons: Aldotetrose
(iii)Five carbons:Aldopentose
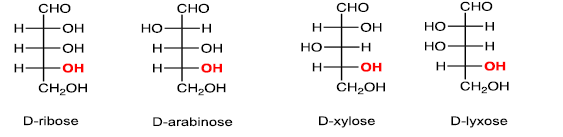
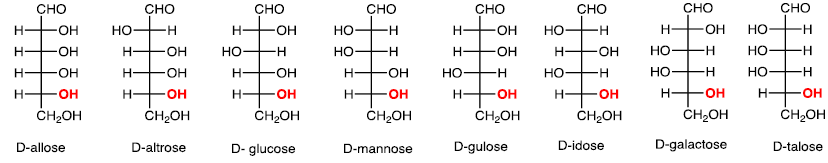
(iv) Six carbons: Aldohexose
(b) Ketoses
Ketoses are less common than aldoses.
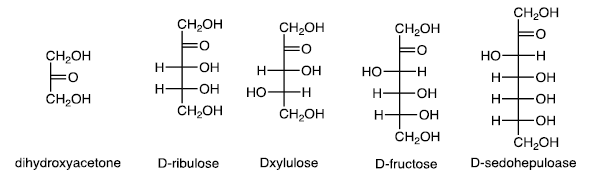
Fructofuranose and Fructopyranose
Cyclic Forms of Carbohydrates: Furanose Forms
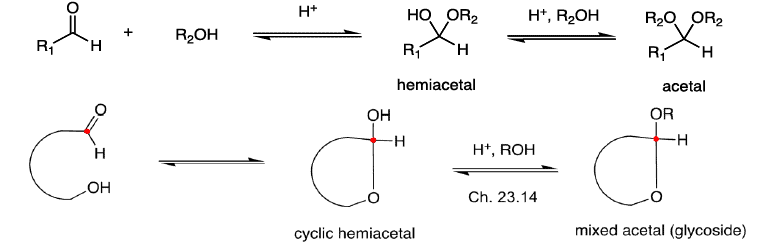
Cyclic Structure of Monosaccharides Hemiacetal Formation
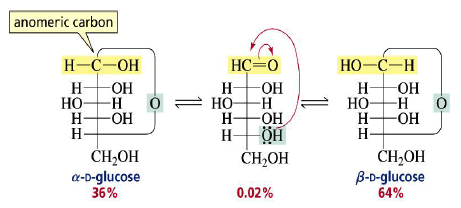
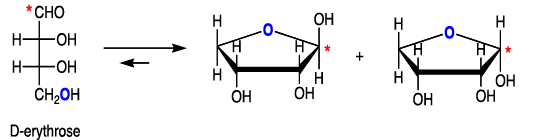
Cyclization of carbohydrates to the hemiacetal creates a new chiral center. The hemiacetal or hemiketal carbon of the cyclic form of carbohydrates is the anomeric carbon. Carbohydrate isomers that differ only in the stereochemistry of the anomeric carbon are called anomers.
Haworth Projections
The structures of cyclic sugars are best represented by the Haworth projections. Haworth projections allow us to see the relative orientation of the OH groups in the ring.
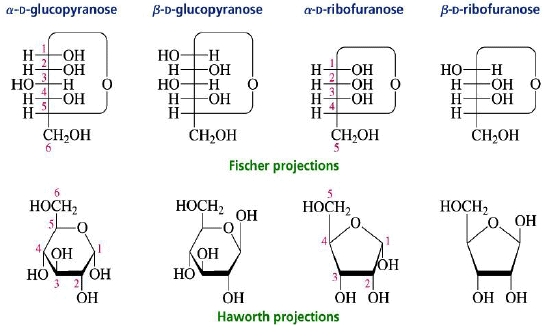
Converting Fischer Projections to Haworth formulas
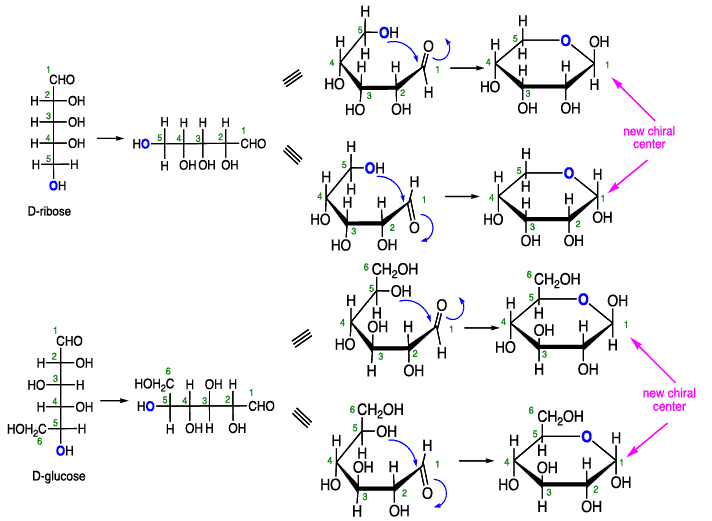
Note: The pyranose forms of carbohydrates adopt chair conformations.
Mutarotation
The α- and β-anomers are in equilibrium, and interconvert through the open form. The pure anomers can be isolated by crystallization.
- When the pure anomers are dissolved in water they undergo mutarotation, the process by which they return to an equilibrium mixture of the anomer.
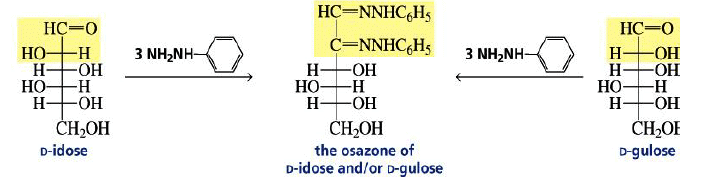
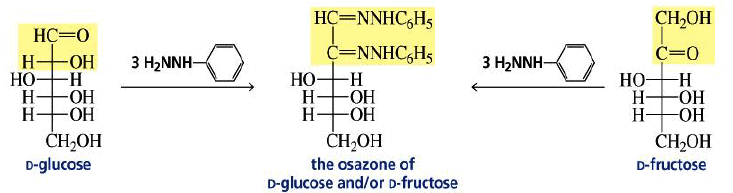
Osazone Formation:
Aldoses and ketoses react with three equivalents of phenylhydrazine.
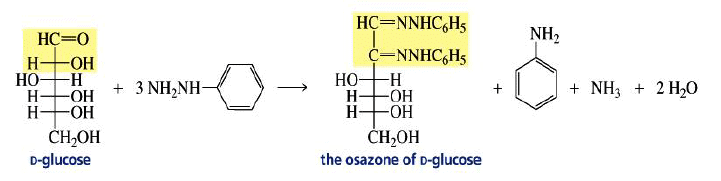
The C-2 epimers of aldoses form identical osazones.
Reaction of Ketoses with Phenylhydrazine.
Deoxy Sugars
Carbohydrates that do not contain hydroxy group.
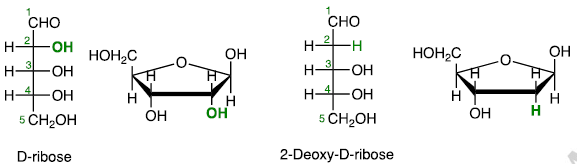
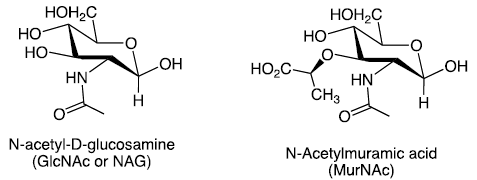
Amino Sugars
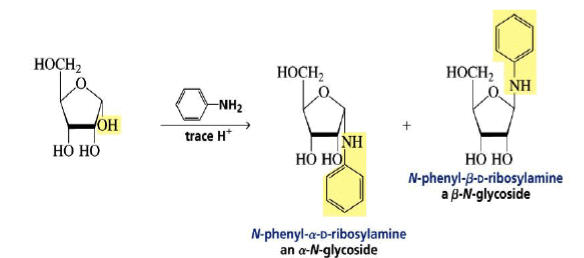
Carbohydrates in which a hydroxyl group is replaced with an —NH2 or —NHAc group
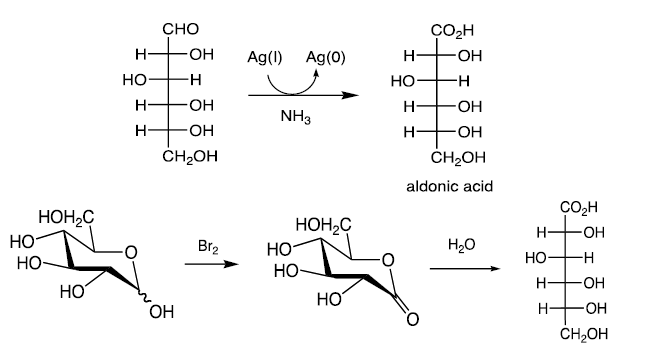
Oxidation of Monosaccharides
C1 of aldoses can be selectively oxidized to the carboxylic acid (aldonic acids) with Br2 or Ag(I) (Tollen’s test).
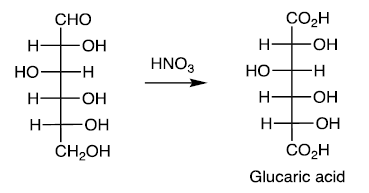
Reducing Sugars
Carbohydrates that can be oxidized to aldonic acids. For example, oxidation of aldoses to aldaric acids with HNO3.
Reduction of Monosaccharides
C1 of aldoses are reduced with sodium borohydride to the 1° alcohol (alditols)
Chain Elongation
The carbon chain of an aldose can be increased by one carbon by Kiliani–Fischer synthesis method.
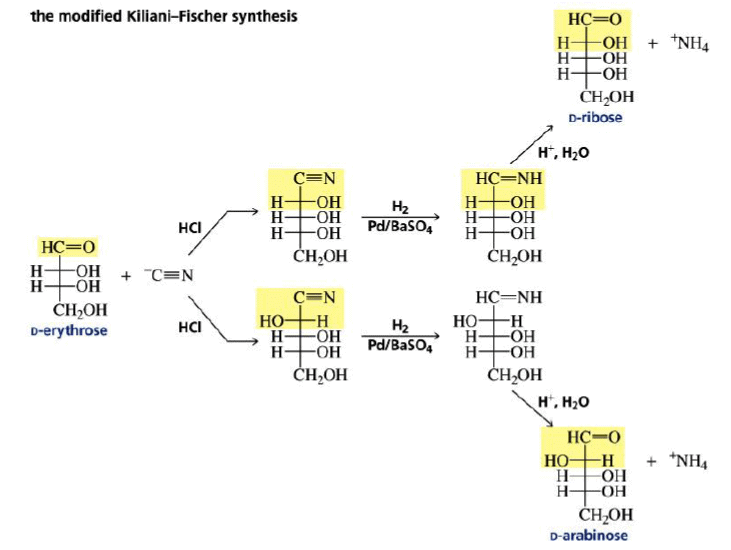
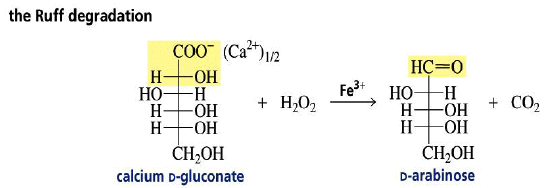
Chain Shortening
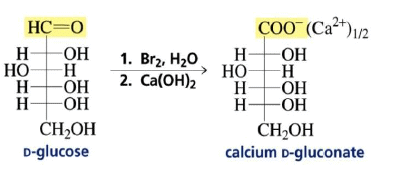
The Ruff degradation shortens an aldose chain by one carbon.
Preparation of the Calcium D-Gluconate for the Ruff Degradation
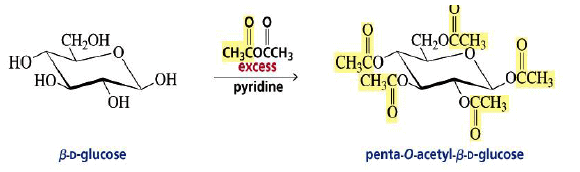
Acylation of Monosaccharides:
The OH groups of monosaccharides show the chemistry of typical alcohols.
Alkylation of the OH Groups
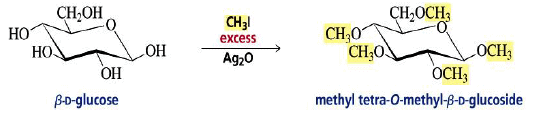
Formation of Glycosides
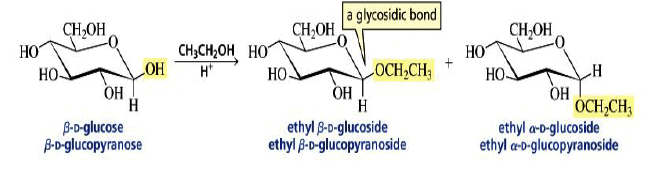
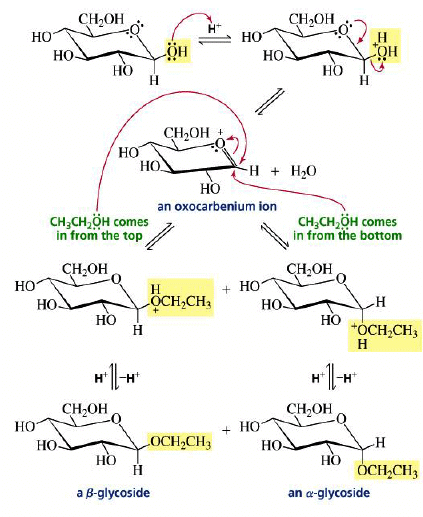
Mechanism:
Formation of an N-Glycoside
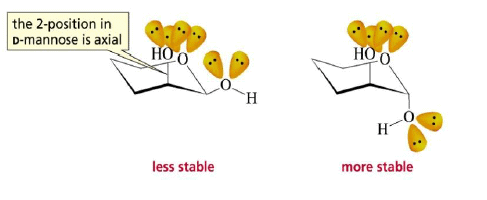
The Anomeric Effect
The anomeric effect refers to the tendency of a group X at C(1) of a pyranose ring to assume the axial rather than the equatorial orientation. This phenomenon is important in carbohydrate chemistry since it influences the composition of isomeric mixtures and hence their reactivities. It has been suggested that the effect is caused mainly by a stabilizing interaction between the axial lone pair of electrons on the ring oxygen atom and the anti-periplanar, antibonding orbital of the C-X bond. This leads to a shortening of the bond between the ring oxygen and the anomeric carbon and a lengthening of the C-X bond. Alternatively, electrostatic repulsive forces between the dipoles due to the ring oxygen lone pairs and the exocyclic oxygen or halogen may account in part for the observed axial preferences.
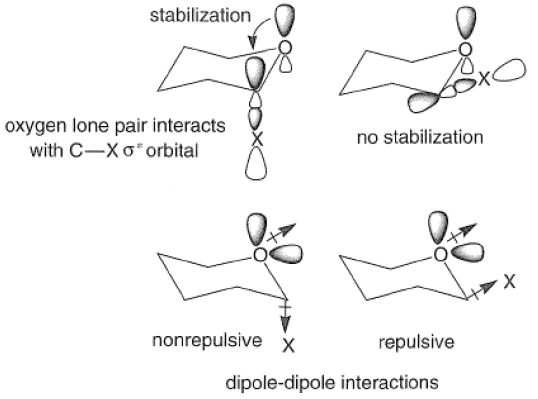
The anomeric effect is solvent and substituent dependent and decreases in the following order: C1> OAc > OMe > OH, as exemplified by the equilibrium concentrations of the alpha and beta anomers of substituted D-glucose in various protic solvents at 250C.
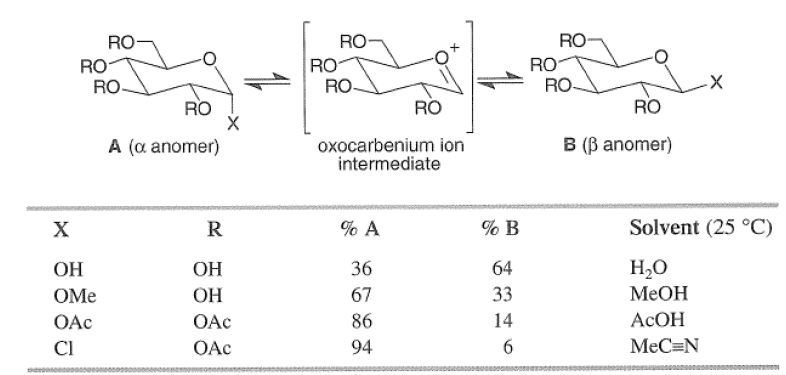
The formation of a glycoside favors the α-glucoside product: the anomeric effect.
|
44 videos|102 docs|52 tests
|
FAQs on Introduction to Biomolecules & Carbohydrates - Organic Chemistry
| 1. What are carbohydrates? |  |
| 2. How are carbohydrates classified based on their behavior on hydrolysis? |  |
| 3. What is the significance of the functional group in classifying carbohydrates? |  |
| 4. How is the number of carbon atoms used to classify carbohydrates? |  |
| 5. What is the significance of cyclic structures in monosaccharides? |  |






















