Mesomeric and Steric Effects | Organic Chemistry PDF Download
Mesomeric Effect
The ability of an atom or group to withdraw or donate π-electrons from an unsaturated system is referred to mesomeric effect.
(a) Electron Donating Mesomeric Effect (+M)
Groups having + M effect releases π-electron towards unsaturated group.
Decreasing order of ‘+M’ effects:
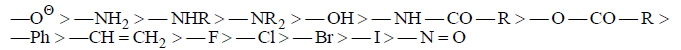
In the periodic table the ‘+M’ effects decreases from left to right in period and decreases from top to bottom:
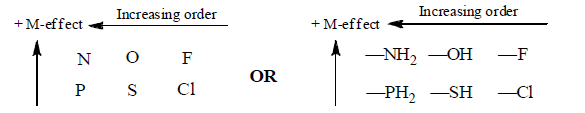
(b) Electron withdrawing Mesomeric effect (–M)
Groups with –M effect have capability to withdraw π-electron from unsaturated system towards itself.
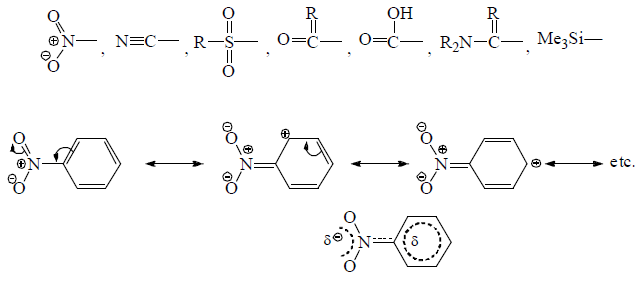
—NO2 withrawing π-electron from aromatic ring.
Applications
(a) Stabilisation of carbocation by mesomeric effect: If α -atom with respect to carbocation has one or more than one lone pair of electron then lone pair of electrons strongly strongly stabilises a carbocation due to the delocalisation of one pair of heteroatom.
Alkoxy and amino groups are important substituents for such tyhpe of carbocations.
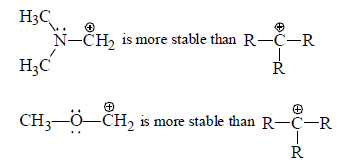
Although these structure have a postive charge on more electrogaive atom, they benefit from an additions bond which satisfies the octet requirement of trivalent carbon.
Stabilising power of some groups which have a least one lone pair in decreasing order is as follows:
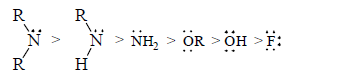 .
.
Electron-withdrawing groups that are substituted directly on the cationic carbon site and have no lone pair of electrons are destabilising.
Destabilising power of some groups indecreasing order is as follows.
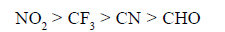
(b) Relative reactivities toward addition of HBr
The relative rates at which alkenes, A, B and C undergo electrophilic addition reaction with a reagent such as HBr demonstrate the effect of delocalized electrons can have on the reactivity of a compound.
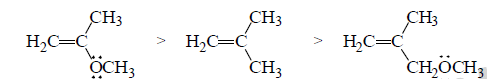
A is the most reactive of the three alkenes. The positive charge of the carbocation intermediate formed in the ratelimiting step is shared by carbon and oxygen. Therefore, it is a more stable carbocation and, therefore, easier to form than the carbocations formed by B and C that have their positive charge localized on a single atom.
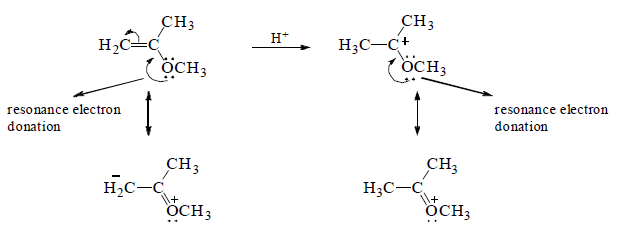
B reacts more rapidly than C with HBr because the carbocation formed by C is destabilized by the OCH3 group that withdrawing electrons inductively (through the σ bonds) from the positivity charged carbon of the carbocation intermediate.
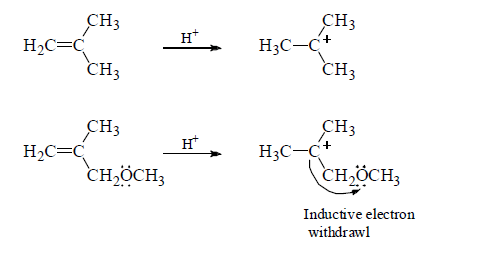
Notice that the OCH3 group in C can only withdraw electrons inductively, whereas the OCH3 group in A is positined so that in additionto withdrawing electrons inductively, it can also donate the lone-pair electrons to stabilize the carbocation. This is called resonance electron donation. Because stabilization by resonacne electron donation
over weighs destabilization by inductive electron withdrawal, the overall effect of the OCH3 group in A is stabilization of the carbocation intermediate.
B. ELECTROMERIC EFFECT (TEMPORARY)
It involves the complete transfer of p-electrons of a multiple bond to one of the bonded atoms in presence of an attacking reagent. As soon as the reagent is removed. The molecule reverts back to its original position.
Steric Effects
These effects are very significant in organic chemistry and biology. Most books would deal with this effect in a very sketchy way, but it is important to understand the basis of this effect. The word steric is derived from ‘stereos’ meaning space. So this effect is manifested when two or more groups or atoms come in close proximity to each other (precisely within each other’s van der Waals radii (definition of van der Waals radii can be found in any standard textbook)) and result in a mutual repulsion. This makes the molecule unstable. The situation can be compared to a crowded bus or train where each passenger stands touching the other one and there is collision, one steps on the other’s feet, hits one another with elbows and so on and so forth. It’s clearly not a very pleasant scenario! It is the same things with molecules. So sheer bulk of the atoms or groups and their proximity can have serious implications. The usual physical clash between groups, almost always is accompanied by an electronic component as well. This is called stereoelectronic effect, which is not the same as the electronic effects discussed above and does not carry have an effect on some other part of the molecule like inductive and resonance effects. When the two atoms get to close, into each other’s van der Waal’s radii, the electron cloud surrounding each atom repel each other leading to a lot of destabilization. Steric effect affects different properties of molecules, like acidity, basicity and general reactivity. In biological systems where everything occurs in the level of angstroms and in a very precise manner, steric effects even due to tiny H atoms can result in the improper folding of proteins, leading to serious diseases like Alzheimer’s Disease.1 Steric clashes can lead to improper DNA replication resulting is destruction of genetic information and hence to a plethora of genetic diseases including cancer.2
Application of Steric Effects
On account of the presence of bulkier groups at the reaction centre, they cause mechanical interference and with the result the attacking reagent finds it difficult to reach the reaction site and thus slows down the reaction. This phenomenon is called steric hinderance or steric effect.
(1) Tertiary alkyl halides having bulky groups form tertiary carbocation readily when hydrolyzed because of the presence of the three bulky groups on the carbon carrying halogen.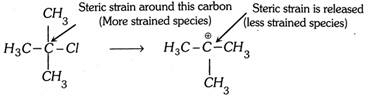
(2) Primary alkyl halide having quaternary carbon does not form a transition state because of the steric strain around alpha carbon by the beta carbon. To release the strain it converts into carbocation. 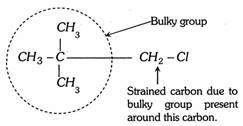 3) Steric strain inhibits the resonance. This phenomenon is known as steric inhibitions of resonance.
3) Steric strain inhibits the resonance. This phenomenon is known as steric inhibitions of resonance.
|
39 videos|92 docs|46 tests
|
FAQs on Mesomeric and Steric Effects - Organic Chemistry
| 1. What is the mesomeric effect? |  |
| 2. How does the mesomeric effect influence the stability of molecules? |  |
| 3. What are steric effects? |  |
| 4. How do steric effects impact the conformation of molecules? |  |
| 5. What are some applications of steric effects in organic chemistry? |  |

















