Elimination Reactions and Molecular Rearrangement -Reaction Mechanism Chemistry | Organic Chemistry PDF Download
| Table of contents |

|
| Elimination Reactions |

|
| E1 Mechanism |

|
| E2 Mechanism |

|
| E1 cB Mechanism |

|
| Molecular Rearrangements |

|
Elimination Reactions
When two groups or atoms from adjacent carbons are eliminated with the formation of unsaturated compounds (alkene or alkyne), the reaction is called elimination reaction. Most commonly a nucleophile (from the α-carbon) and a proton from the β-carbon are eliminated. Hence, the reaction is known as 1, 2- or -elimination αβ or simply -elimination.
Some familiar elimination reactions are:
(i) Dehydrohalogenation of alkyl halides by base.
(ii) Dehydration of alcohols by acids.
(iii) Hofmann’s degradation of quarterney bases by heat.
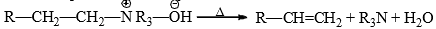
The presence of at least one hydrogen on the β-carbon is necessary for elimination.
The driving forces for elimination are :
(a) stability of the olefin formed
(b) the relief from steric strain due to crowding in the substrate.
Branching at the β-carbon (as also at the α-carbon ) of the substrate produces substituted olefins stabilized by hypyper conjugation and hence favours elimination. Thus, Me3CCl gives only 16% olefin while Me2CH—CMe2Cl gives 62% olefin.
Strain in the substrate due to crowding the substituents can be relieved on the formation of olefin since the bond angles increase from 109.5° in the substrate (sp3-hydridized) to 120° in the product (sp2-hybridized). Hence 3° halides favour elimination most and 1° halides the least, i.e., the order of elimination in halides is 3° > 2° > 1°.
The elimination reactions like SN reactions may proceed by either unimolecular. These elimination reactions have been designated E1 and E2 on their resemblance to SN1 mechanism and SN2 mechanism respectively.
E1 Mechanism
The elimination reaction in which the rate of reaction is dependent on the concentration of the substrate only, i.e., kinetically of the first order, is designated E1. Since the rate of reaction is independent of the concentration of the reagent, it is interpreted, as in SN1, that the first step of the reaction is the slowest step involving ionization of the substrate. This is followed by the rapid removal of a proton from the β-carbon by the reagent in the second step. 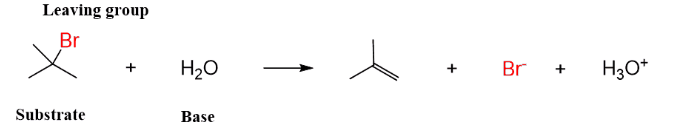
This is illustrated below.
(i) Dehydrahalogenation of alkyl halides: The rate of elimination of a halacid from t-butyl bromide in basic medium is found to be proportional to [Me3CBr]. Therefore, the halide undergoes slow ionization in the first step. This is followed by a rapid extraction of a proton from the carbocation by the base or solvent in the second step.
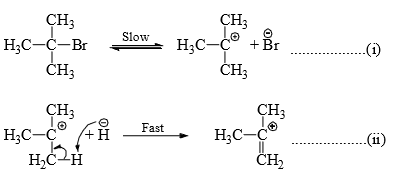
it is seen that a carbocation is formed in the first step in both E1 and SN1 reactions. Hence, the reagent can attack the carbon to given substitution product and also can accept a proton to give elimination product. In practice both alcohol (substitution product) and alkene (elimination product) are obtained on hydrolysis of Me3CBr.
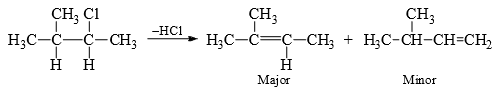
When more than one alkene can be formed, that alkene will predominate which has larger number of alkyl groups on the double-bonded carbons-this is Saytzev’s rule. This is understandable since the substituted alkyl group will stabilize the alkene by hyperconjugation.
(ii) Dehydration of alcohols—Acid-catalysed dehydration of alcohols follows E1 mechanism. The dehydration is carried out at elevated temperature with sulphuric acid. Alcohol is first protonated which weakens the C—O bond. Hence, water is eliminated from the protonated alcohol with generation of a carbocation. The latter then expels a proton to form a stable alkene.
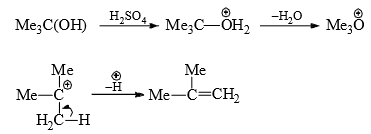
The concentration of acid depends upon how fast the carbocation is formed, i.e., on the stability of the carbocation. primary alcohols generate unstable carbocations and hence require high temperature and high concentration of acid while 3° alcohols require lower temperature and lower concentration of acid for dehydration. Thus,
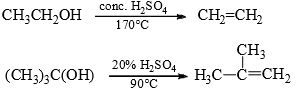
E1 reaction is facilitated by
(i) Branching at the α and β -carbons of the substate-for stability of the olefin.
(ii) Strong polar solvent-to aid ionisationi,
(iii) Low concentration of base-the greater stability of the alkene over the carbocation makes the extraction of proton easy.
E2 Mechanism
When the rate of elimination reaction is dependent on both the substrate and the reagent, i.e., rate ∝ [Substrate][reagent], the reaction is kinetically of the second order or bimolecular. Such bimolecular elimination reaction is designated E2 on its similarity with SN2.
Since the reaction involves both the reactants in the rate-determining step, it is interpreted as in SN2, that the reaction occurs in one step in which both the groups (proton and the leaving group) depart simultaneously through the intermediate transition state. It is visualized that as the base abstracts a proton from the β-carbon, simultaneous departure of the nucleophile takes place from the α-carbon. In the transition state the βC — H and αC — X bonds are stretched on the attack of the reagent with the incipient π-bond formation.
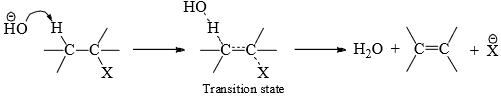
The energy of the transition state will be least when the two leaving groups, the α-and β-carbons and the attacking base are coplanar in the transition state. Also, the two leaving groups (H and X) should be trans to each other to effect π bond.
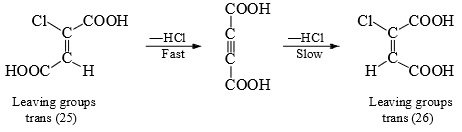
The two leaving groups orient themselves in the trans position when a σ bond exists between the α-and β-carbons . When, however, free rotation is not allowed as in the case of double bond, elimination is difficult when the leaving groups are cis to each other. Thus, acetylene dicarboxylic acid is more easily formed from chlorofumaric acid (25) than from chloromaleic acid (26).
E2 reaction is facilitated by
(i) Branching at α-and β-carbons —since more stable olefin is formed.
(ii) Strong base of high concentration—since a strong C—H bond has to break,
(iii) Solvant of low polarity—polar solvents form a strong solvent wall around the base restricting the attack. Hence DMF or DMSO are usually used as solvents.
E1 cB Mechanism
It may be argued that a second-order elimination reaction may as well proceed in two steps as in E1 reaction. The first step involves a fast and reversible removal of a proton from the β-carbon with the formation of a carbanion which then loses the leaving group in the second slow rate-determinig step.
The overall rate of this reaction is thus dependent on the concentration of the conjugate base of the substrate (carbanion). Hence, this mechanism has been designated as E1 cB (Elilmination, Unimolecular from conjugate base).
To distinguish between E2 and E1cB mechanisms, deuterium exchange experiment was perfomed. for this 2-phenylethyl bromide was treated with sodium ethoxide in EtOD. This substrate was selected because the Ph group is expected to increase the acidity of the β-hydrogen and also to stabilize the carbanion to exist long enough for incorporation of deuterium in the starting material from the solvent EtOD.
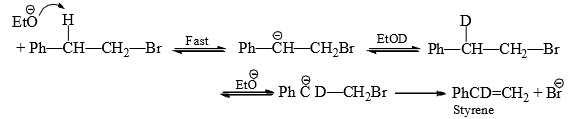
The reaction was interrupted before completion and analysed for deuterium content. No deuterium incorporation was found either in the substrate or in the styrene. Hence, no reversible carbanion was formed. The reaction followed E2 path. However, the E1cD mechanism does operate in substrates having strong electron-withdrawing groups, e.g., chlorine on β-carbon, and poor leaving groups, e.g., fluorine as in Cl2CH—CF3.
Orientation in elimination reactions: Substrates having alternative β-hydrogen give a mixture of olefins on elimination.
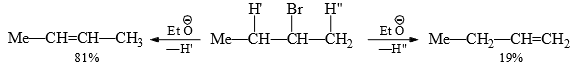
To help in forecasting the major product of elimination (alkene), there are two empirical rules:
(i) Saytzev Rule: The rule states that neutral substrates (alkyl halides, alkyl toluenesulphonates) lead predominantly depends wholly on the relative stabilities of the olefins. Therefore, Saytzev rule governs the orientation of E1 reaction.
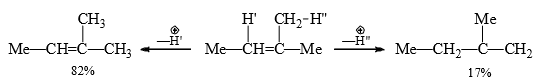
In suitable substrates the rearrangement of the carbocation before elimination may give different alkenes. For steric reasons the non-Saytzev product may be the major product in suitable substrates.
The olefin obtained through path (b) is minor due to steric hindrance. In E2, it is suggested that the incipient olefinic bond formation in the transition state is being stabilized by the inductive effect of the alkyl groups, thereby lowering the energy of the transition state. Therefore, with the increasing number of alkyl groups there will be increasing stability of the transition state with progressive lowering of energy of the transition state.
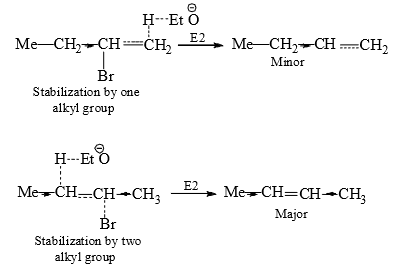
(ii) Hofmann Rule: When a quanternary ammonium hydroxide is strongly heated (<125°C) it decomposes to yield a tertiary amine, water and alkene.
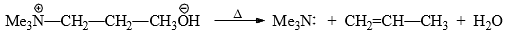
This is known as Hofmann elimination or β-elimination. The reaction involves abstraction of a proton from β-carbon with the simultaneous expulsion of the leaving group.
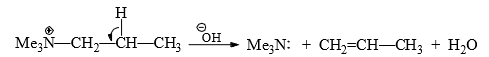
When there are alternative β-hydrogens inthe quaternary ammonium salts, a mixture of alkenes is formed. Hofmann rule states that in case of alternative β-hydrogens in the charged substrates (quaternary ammonium and sulphonium salts), the least substituted alkene is predominantly formed.
Thus, 2-butylquaternary ammonium hydroxide undergoes Hofmann elimination to give 1-butene as the major product.
To explain, it has been suggested that the strong electron-withdrawing effect of Me3N+ group makes the hydrogens of the β-carbons more acidic for facile abstraction by the base and stabilizes the incipient carbanion formation in the transition state on gradual stretching of the βC — H bond. In this particular compound with alternative β-hydrogens,and "β-hydrogens are less acidic due to +1 effect of the adjacent methyl group.
Hence, 'β-hydrogen which is relatively more acidic is removed to give predominantly I-butene.
Hofmann product increases on increasing in the base. The steric effect due to crowding in the leaving group or in the substrate promotes Hofmann elimination.
In general the proton acidity and for that matter the inductive effect is the more important factor for Hofmann elimination. Whatever be the conditions, that alkine is formed in which its double bond is conjugated with Ph or C=C groups, e.g.,
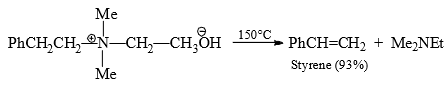
Molecular Rearrangements
In most organic reactions the functional group of the substrate undergoes structural change without affecting the carbon skeleton of the molecule. There are however many other organic reactions in which atoms, groups (alkyl or aryl), double bonds or dunctional groups migrate within the molecule. The latter types of reactions are known as rearrangement reactions or molecular rearrangement.
Thus, molecular rearrangement involves modification in the sequence of atoms or groups in a molecule resulting in a new structure. Migration of atoms or groups occurs from one atom to another (usually adjacent) within the same molecule by Whitmore, 1, 2-shift.
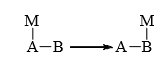
The atom A is called the migration origin and the atom B is called the migration terminus. The shift of M from A to B is called 1, 2-shift. When M is hydrogen, alkyl or aryl group, it is called 1, 2-hydride shift, 1, 2-hydride shift, 1, 2 alkyl shift or 1, 2-aryl shift respectively. The migration group may move with its bonding pair of electrons (anionotropic), without the bonding pair (cationotropic) or with a single electron (free radical). This means that rearrangement may take place through intermediates that are cations, anions or free radicals. A large number of molecular rearrangements involve anionotropic migration of a group to an adjacent atom with incomplete octet (i.e., electron-deficient). The rearrangement involving electron-deficient species are more common.
Rearrangement due to migration to electron-deficient atom (C, N, O)
During a reaction an electronegative group may depart with its bonding pair of electrons leaving behind an electrondeficient atom with six electrons. This results in the migration (1, 2-shift) of a group with its bonding pair from the adjacent atom tot he electron-deficient atom. The migrating group may be hydrogen, carbon, nitrogen, oxygen, sulphur or halogens.
The migrating group may either
(i) detach from the migration origin and then form bond with the migration terminus (electron-deficient atom)
(ii) may remain partly bonded to both migration origin and terminus to form a bridged ion intermediate or transition state. The general mechanistic possibilities by 1, 2-shift are given below.
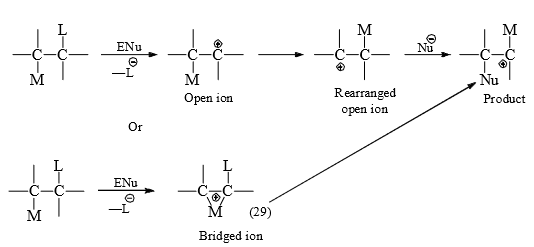
When M is a hetero atom, besides crossover experiments there is stereochemical evidence that (29) is formed. The nonbonding electrons of the hetero atom are said to be involved in the neighbouring group participation in forming (29) since that of reaction is increased by anchimeric* assistance.
Alkyl group or hydrogen has no non-bonding electrons. It is quite possible that those first leave the migration origin to form open ion and then form the bridged ion becuase the rearrangemennt do not show the increased rate expected from anchimeric assistance.
The corresponding rearrangements with radical and anion intermediates are seldom encountered because of unfavourable distribution of electrons in the molceular orbital encompassing the three atoms in the bridged ion. Rearrangements to electron-deficient carbon, nitrogen and oxygen will be separately studies.
|
44 videos|102 docs|52 tests
|
FAQs on Elimination Reactions and Molecular Rearrangement -Reaction Mechanism Chemistry - Organic Chemistry
| 1. What is the E2 mechanism in elimination reactions? |  |
| 2. How does the E1cb mechanism differ from the E2 mechanism? |  |
| 3. What factors influence the rate of elimination reactions? |  |
| 4. What are molecular rearrangements in the context of elimination reactions? |  |
| 5. How do stereochemistry and regioselectivity play a role in elimination reactions? |  |
















