Le-Chatelier’s Principle & Factors Affecting Equilibrium Constant (K) | Physical Chemistry PDF Download
11. Disturbance in equilibrium: The Le-Chatelier’s Principle
This principle, which is based on the fundamentals of a stable equilibrium, states that “When a chemical reaction at equilibrium is subjected to any stress, then the equilibrium shifts in that direction in which the effect of the stress is reduced”.
Confused with “stress” Well by stress here what we mean is any change of reaction conditions e.g. in temperature, pressure, concentration etc.
This statement will be explained by the following example.
Let us consider the reaction: 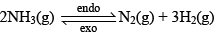
Let the moles o f N2, H2 and NH3 at equilibrium be a, b and c moles respectively. Since the reaction is at equilibrium,
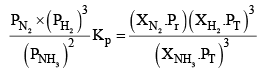
Where,
X terms denote respective mole fractions and PT is the total pressure of the system.
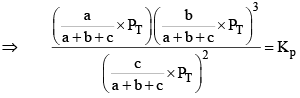
Here,
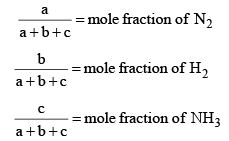
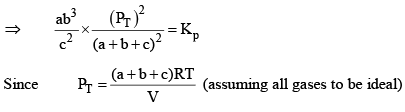
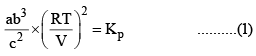
Now, let us examine the effect of change in certain parameters such as number of moles. Pressure, temperature etc.
If we increase a or b, the left hand side expressio n becomes QP (as it is disturbed from equilibrium) and we can see that QP > KP
The reaction therefore moves backward to make QP = KP
If we increase c, QP < KP and the reaction has to move forward to revert back to equilibrium.
If we increase the volume of the container (which amounts to decreasing the pressure), QP < KP and the reaction moves forward to attain equilibrium.
If we increase the pressure of the reaction, then equilibrium shifts towards backward direction since in reactant side we have got 2 moles and on product side we have got 4 moles. So pressure is reduced in backward direction.
However from the expression if we increase the temperature of the reaction, the left hand side increase (QP) and therefore does it mean that the reaction goes backward (since QP > KP)
Does this also mean that if the number of moles of reactant and product gases are equal no change in the reaction is observed on the changing temperature (as T would not exist on the left hand side)?. The answer to these quest ions is No. This is because KP also change with temperature. Therefore we need to know the effect of temperature on both QP and KP to decide the course of the reaction.
1. Effect of Concentration on Equilibrium:
If the concentration of a component is increased, reaction shifts in a direction which tends to decrease its concentration
For exmaple: 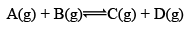
At equilibrium: Moles 1 1 1 1 KC = 1
If added 1 mole of D at equilibrium:
1 1 1 1 QC = 2
QC> KC react ion implies reaction proceeds in backward direct ion.
At new equilibrium: 1 +x 1+ x 1-x 2-x
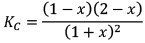
Note: Here KC will remain constant even after addition of D Because KC depends on temperature only.
2. Effect of change of pressure / volume on equilibrium
Three cases arises
· Δng = 0
· Δng > 0
· Δng < 0
Case I:Δng = 0
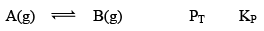
At equilibrium 1 atm 1 atm 2 atm 1
When we double the pressure or half the volume at equilibrium at t
2 atm 2 atm 4 atm 1
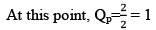
Here, KP = QP
Hence reaction will not move in any direction. It resembles the reaction is still at equilibrium.
Case II: Δng> 0
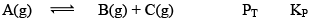
At equilibrium 1 atm 1 atm 1 atm 3 1
When we double the pressure or half the volume
At t 2 atm 2 atm 2 atm 6 2
At this time, 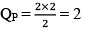
QP = 2
QP>KP; hence reaction will move in backward direction.
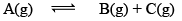
At new equation 2+P 2-P 2-P
Case III:Δng < 0
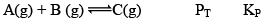
At t = teq 1 atm 1 atm 1 atm 3 1
When we double the pressure or half the volume at equilibrium
At t 2 atm 2 atm 2 atm 6 2
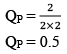
Since, QP< KP React ion will move in forward direction
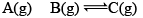
New equilibrium 2–P 2–P 2+P
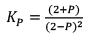
Table to calculate

3. Effect of Temperature on Equilibrium:
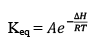
Now we will derive the dependence of KP on temperature.
Starting with Arrhenius equation of rate constant

Where, kf = rate constant for forward reaction, Af = Arrhenius constant of forward reaction,
Eaf = Energy of activation of forward reaction

Dividing (i) by (ii) we get,
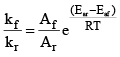
We know that 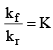 (equilibrium constant)
(equilibrium constant)
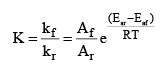
At temperature T1

At temperature T2
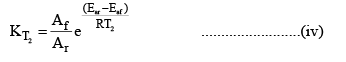
Dividing (iv) by (iii) we get
The enthalpy of a reaction is defined in terms of activation energies as 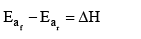
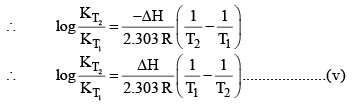
For and exothermic reaction, ΔH would be negative. If we increase the temperature of the system (T2 > T1), the right hand side of the equation (V) becomes negative.
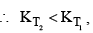
that is, the equilibrium constant at the higher temperature would be less than that at the lower temperature.
Now let us analyse our question. Will the reaction go forward or backward?
Before answering this, we must first encounter problem. If temperature is increased. The new KP would either increase or decrease or remain unchanged. If Kp increase and QP decreases.
Then 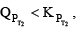 therefore the reaction moves forward. If Kp increase and QP remains same then
therefore the reaction moves forward. If Kp increase and QP remains same then 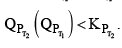 Again, the reaction moves forward. What, if KP increase and QP also increase?
Again, the reaction moves forward. What, if KP increase and QP also increase?
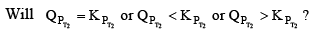 This can be answered by simply looking at the dependence of QP and KP on temperature. You can see from the equation that KP depends on temperature exponentially. While Q’s dependence on T would be either to the power g, l, t………..Therefore the variat ion in KP would still be greater QP and the reaction moves forward again. Therefore, to see it decreases, reaction moves backward and if it remains fixed, then no change at all.
This can be answered by simply looking at the dependence of QP and KP on temperature. You can see from the equation that KP depends on temperature exponentially. While Q’s dependence on T would be either to the power g, l, t………..Therefore the variat ion in KP would still be greater QP and the reaction moves forward again. Therefore, to see it decreases, reaction moves backward and if it remains fixed, then no change at all.
Therefore, two cases arises:
(1) ΔH is +ve (Endothermic Reactions)
(2) ΔH is -ve (Exothermic Reactions)
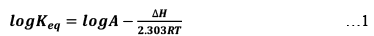
Endothermic Reaction:
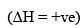
As we increase temperature, it is clearly understood by equation (1). That Keq also increase. Hence, with increase in temperature Keq increases in endothermic reactions.
Reactions will shift in forward direction.
Exothermic Reaction (ΔH = -ve)
As temperature is increased, the magnitude o f Keq decreases. Hence, increase in temperature leads to decrease in Keq. React ion will shift in backward direction.
4. Effect of addition of inert gases to a reaction at equilibrium
1. Addition at constant pressure
Let us take a general reaction
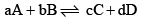
We know,
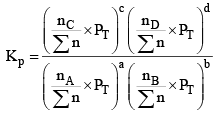
Where,
nC , nD , n A , n B denotes the no. of moles of respective components and PT is the total pressure and ∑n = total no. of moles of reactants and products.
Now, rearranging, 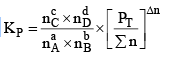
Where Δn = (c + d) - (a + b)
Now, Δn can be = 0, < 0 or > 0
Let us take each case separately.
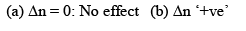
Addition of inert gas increases the 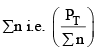 is decreased and so is
is decreased and so is 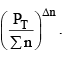
So products have to increase and reactants have to decrease to maintain constancy of KP. So the equilibrium moves forward.
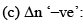
In this case 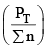 decreases but
decreases but 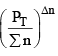 increases. So products have to decrease and reactants have to increase to maintain constancy of KP. So the equilibrium moves backward.
increases. So products have to decrease and reactants have to increase to maintain constancy of KP. So the equilibrium moves backward.
2. Addition at Constant Volume:
Since at constant volume, the pressure increases with addition of inert gas and at the same time ∑n also increases, they almost counter balance each other. So 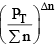 can be safely approximated as constant. Thus addition of inert gas has no effect at constant volume.
can be safely approximated as constant. Thus addition of inert gas has no effect at constant volume.
|
84 videos|142 docs|67 tests
|
FAQs on Le-Chatelier’s Principle & Factors Affecting Equilibrium Constant (K) - Physical Chemistry
| 1. What is Le-Chatelier's Principle? |  |
| 2. How does Le-Chatelier's Principle apply to changes in temperature? |  |
| 3. What effect does a change in pressure have on an equilibrium system? |  |
| 4. How does Le-Chatelier's Principle explain changes in concentration? |  |
| 5. How can catalysts affect the equilibrium constant (K)? |  |





















