Short & Long Answer Questions: Aldehydes, Ketones & Carboxylic Acids | Chemistry for JEE Main & Advanced PDF Download
Q.1 Although phenoxide ion has more no. of resonating structures than carboxylate ion, even though carboxylic acid is a stronger acid why?
Ans:- The phenoxide ion has non-equivalent resonance structures in which –ve charge is
at less electronegative C atom and +ve charge as at more electronegative O-atom.
In carboxylate ion –ve charge is delocalized on two electronegative O-atoms hence
resonance is more effective and a stronger acid.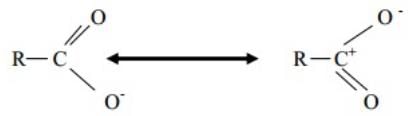 Q.2 Why Carboxylic acid have higher boiling point than alcohols as alcohol forms strongest inter molecular hydrogen bonding?
Q.2 Why Carboxylic acid have higher boiling point than alcohols as alcohol forms strongest inter molecular hydrogen bonding?
Ans. As Carboxylic acid forms a dimer due to which its surface area increases and
forms strong intermolecular H-bonding. It has more boiling point than alcohol.
Q.3 Why does solubility decrease with increasing molecular mass in carboxylic acid?
Ans. Because of the increase in alkyl chain length which is hydrophobic in nature. Hence solubility decreases.
Q.4 Why aldehydes are more reactive than ketones when undergo nucleophilic addition reaction?
Ans. a. + I effect:- The alkyl group in Ketones due to their e-releasing character decrease the +ve charge on C-Atom and thus reduce its reactivity.
b. Steric hinderance:- Due to steric hinderance in ketones they are less reactive.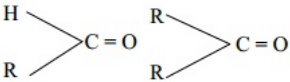
Q.5 Why PCC cannot oxidise methanol to methanoic acid and while KMNO4 can?
Ans. This is because PCC is a mild oxidising agent and can oxide methanol to methanal
only. While KMNO4 being strong oxidising agent oxidises it to methanoic acid.
Q.6 During preparation of esters from a carboxylic acid and an alcohol in the presence of acid catalyst water or ester formed should be removed as soon as it is formed.
Ans. The formation of esters from a carboxylic acid and an alcohol in the presence of acid catelyst in a reversible reaction.
R – COOH + R′OH  H2SO4 R – COOR′ + H2O
H2SO4 R – COOR′ + H2O
To shift the equilibrium forward, the water or ester formed should be removed as fast as it is formed.
Q.7 Why HCOOH does not give HVZ reaction while CH3COOH does?
Ans. CH3COOH contains α-hydrogens and hence give HVZ reaction but HCOOH does not
contain α-hydrogen and hence does not give HVZ reaction.
Q.8 Suggest a reason for the large difference in the boiling point of butanol and butanal although they have same solubility in water.
Ans. Because Butanol has strong intermolacular H-bonding while butanal has weak dipole-dipole interaction.
However both of them form H-bonds with water and hence are soluble.
Q.9 Would you expect benzaldehyde to be more reactive or less reactive in nucleophilic addition reaction than propanol. Explain.
Ans. C-atom of Carbonyl group of benzaldehyde is less electrophilic than C atom of Carbonyl group in propanol. Polarity of Carbonyl group is in benzaldehyde reduced due to resonance making it less reactive in nucleophillic addition reactions.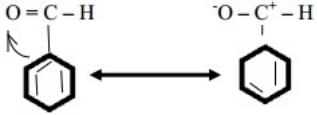 Q.10 Why does methanal not give aldol condensation while ethanol gives?
Q.10 Why does methanal not give aldol condensation while ethanol gives?
Ans. This is because only those compounds which have α-hydrogen atoms can undergo aldol reaction ethanol possess α-hydrogen and undergoes aldol condensation. Methanal has no alpha hydrogen atoms hence does not undergo aldol condensation.
Q.11 Why does methanal undergo Cannizaro’s reaction?
Ans. Methanal undergo Cannizaro’s reaction because it does not possesses α-hydrogen atom.
Q.12 Which acid is stronger and why?
F3C-C6H4COOH and CH3C6H4COOH
Ans. CH-3 has strong (-I) effect Whereas, CF-3 has strong (+I)effect
Due to greater stability of F3CC6H4COO- ion over CH3-C6H4COO- ion.
CF3 C6H4COOH is much stronger acids than CH3-C6H4COOH.
Q.13 Explain why O-hydroxy benzaldehyde is a liquid at room temperature while p hydroxy benzaldehyde is a high melting solid.
Ans. Due to intramolecular H-bonding in O-hydroxy benzaldehyde exists as discrete molecule whereas due to intermolecular H-bonding p-hydroxy benzaldehyde exist as associated molecules. To break this intermolecular H-bonds a large amount of energy is needed. Consequently P-isomer has a much higher m.p. and b.p. than that of O-isomer. As a result O-hydroxy benzaldehyde is liquid.
Q.14 Why is the boiling point of an acid anhydride higher than the acid from which it is derived?
Ans. Acid anhydrides are bigger in size than corresponding acids. They have more surface area, more Van der Waals force of attraction hence have higher boiling point.
Q.15 Why do Carboxylic acids not give the characteristic reactions of a carbonyl group?
Ans. Due to resonance, It doesn’t give the characteristics reactions of carbonyl group. It does not have free  Q.16 Cyclohexanone forms cyanohydrin in good yield but 2,2,6 trimethyle cyclo-hexanone does not. Why?
Q.16 Cyclohexanone forms cyanohydrin in good yield but 2,2,6 trimethyle cyclo-hexanone does not. Why?
Ans. In 2,2,6 trimethyl cyclohexanone, there is steric hinderance of 3 methyl groups, It
does not form cyanohydrin in good yield.
Q.17 Why is carboxyl group in benzoic acid meta directing?
Ans. In benzoic acid the Carboxyl group is meta directing because it is electron withdrawing. There is +ve charge on ortho acid para positions. Electrophillic substitution takes place at meta-position.
Q.18 Treatment of Benzaldehyde with HCN gives a mixture of two isomers which cannot be separated even by careful fractional distillation. Explain why?
Ans. It is because we get two optical isomers which have same physical Properties cannot be Separated by Fractional distillation.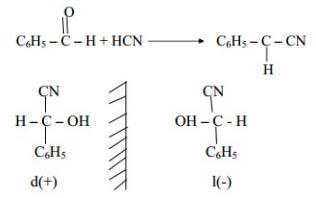
Q.19 Sodium Bisulphite is used for the purification of aldehydes and Ketones. Explain.
Ans. Aldehydes and Ketones form addition compounds with NaHSO3 whereas impurities do not. On hydrolysis we get pure aldehydes and Ketones back.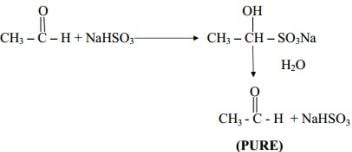
Q.20 Why pH of reaction should be carefully controlled while preparing ammonia derivatives of carbonyl compound?
Ans. In strongly acidic medium ammonia derivatives being basic will react with acids and will not react with carbonyl compound. In basic medium, OH will attack carbonyl group.pH of a reaction should be carefully controlled.
Q.21 Why formic acid is stronger acid than acetic acid?
Ans. Due to +I effect, CH3- group in acetic acid increases e- density on carbon atom which makes it. While in formic acid no such pushing group is present, hence is more stronger acid than acetic acid.
Q.22 Why is oxidation of alcohols to get aldehydes carried out under controlled conditions?
Ans. It is because aldehydes get further oxidised to acids, oxidation of alcohols to aldehydes needs to be controlled.
Q23. Why the oxidation of toluene to benzaldehyde with CrO3 is carried out in the presence of acetic anhydride.
Ans. If acetic anhydride is not used we will get benzoic acid. Acetic anhydride used to prevent oxidation of benzaldehyde to benzoic acid.
Q.24 Melting point of an acid with even no. of carbon atoms is higher than those of its neighbour with odd no. of carbon atoms.
Ans. They fit into crystal lattice more readily than odd ones that is why they have higher lattice energy and higher melting point.
Q.25 Why do aldehydes have lower boiling point than corresponding alcohols?
Ans. Alcohols have lower boiling point as they are not associated with intermolecular whereas alcohols are associated with intermoleculer H-bonding. Aldehydes have lower boiling point.
Q.26 Why do aldehydes behave like polar compounds?
Ans. 
Q.27 Most aromatic acids are solids while acetic acid and others of this series are liquids. Explain why?
Ans. Aromatic acids have higher molecular weight and more van-der waals force of attraction as compared to aliphatic acids They are solids.
Q.28 Ethers possess a dipole moment ever if the alkyl radicals in the molecule are identical. Why?
Ans. It is because ethers are bent molecules, dipole do not get cancelled.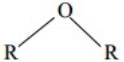
Q.29 Why does acyl chlorides have lower Boiling point than corresponding acids?
Ans. Acyl chlorides are not associated with intermolecular H-bonding. They have lower boiling point.
Q.30 Why ethers are stored in coloured bottles?
Ans. They are stored in coloured bottles. In presence of sunlight they react with oxygen to form peroxides which may cause explosion.
Q.31 Why formaldehyde cannot be prepared by Rosenmund’s reduction?
Ans. Because the formyl chloride thus formed is unstable at room temperature. Cannot be prepared by Rosenmund reduction.
Very Short Answer Type Questions
Q.1 Name the reaction and the reagent used for the conversion of acid chlorides to the
corresponding aldehydes.
Ans. Name: Rosenmund‘s reaction, Reagent: H2 in the presence of Pd (supported over
BaSO4 ) and partially poisoned by the addition of Sulphur or quinoline.
Q.2 Suggest a reason for the large difference in the boiling points of butanol and butanal, although they have same solubility in water.
Ans. The b.p. of butanol is higher than that of butanal because butanol has strong
intermolecular H-bonding while butanal has weak dipole-dipole interaction. However
both of them form H-bonds with water and hence are soluble.
Q.3 What type of aldehydes undergo the Cannizaro reaction?
Ans. Aromatic and aliphatic aldehydes which do not contain α- hydrogen.
Q.4 Out of acetophenone and benzophenone, which gives iodoform test? Write the reaction involved. (The compound should have CH3CO-group to show the iodoform test.)
Ans. Acetophenone (C6H5COCH3 ) contains the grouping (CH3CO attached to carbon) and hence given iodoform test while benzophenone does not contain this group and hence
does not give iodoform test.
Q.5 Give Fehling solution test for identification of aldehyde gp (only equations). Name the aldehyde which does not give Fehling‘s soln. test.
Ans. R — CHO — 2 Cu 2+ + 5 OH – → RCOO– + Cu2O + 3 H2O Benzaldehyde does not give Fehling soln. test. (Aromatic aldehydes do not give this test.)
Q.6 What makes acetic acid a stronger acid than phenol?
Ans. Greater resonance stabilization of acetate ion over phenoxide ion
Q.7 Why HCOOH does not give HVZ (Hell Volhard Zelinsky) reaction but CH3COOH does?
Ans. CH3COOH contains α- hydrogen and hence give HVZ reaction but HCOOH does not
contain α-hydrogen and hence does not give HVZ reaction.
Q.8 During preparation of esters from a carboxylic acid and an alcohol in the presence of an acid catalyst, water or the ester formed should be removed as soon as it is formed.
Ans. The formation of esters from a carboxylic acid and an alcohol in the presence of
acid catalyst in a reversible reaction.
To shift the equilibrium in the forward direction, the water or ester formed should be
removed as fast as it is formed.
Q.9 Arrange the following compounds in increasing order of their acid strength. Benzoic acid, 4-nitrobenzoic acid, 3, 4-dinitrobenzoic acid, 4-methoxy benzoic acid.
Ans. 4-methoxybenzoic acid < benzoic acid < 4-nitrobenzoic acid <4, dinitrobenzoic acid.
Q. 10 How is tert-butyl alcohol obtained from acetone?
Ans. 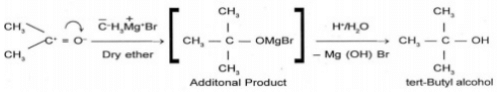
Short Answer Type Questions
Q.1 Arrange the following compounds in increasing order of their boiling points. Explain by giving reasons.
CH3CHO, CH3CH2OH, CH3OCH3, CH3CH2CH3.
Ans. The molecular masses of all these compounds are comparable:
CH3CHO (44), CH3CH2OH (46), CH3COCH3(46), CH3CH2CH3 (44).CH3CH2OH exists as an associated molecule due to extensive intermolecular hydrogen bonding and hence its boiling point is the highest (351 K). Since dipole-dipole interactions are stronger in CH3CHO than in CH3OCH3, hence boiling point of CH3CHO (293 K) is much higher than that of CH3OCH3(249 K). Further, molecules of CH3CH2CH3 have only weak Vander Waals forces while the molecules of CH3OCH3 have little stronger dipole-dipole interactions and hence the boiling point of CH3OCH3 is higher (249 K) than that of CH3CH2CH3 (231 K). Thus the overall increasing order of boiling points is :
CH3CH2CH3 < CH3OCH3 < CH3CHO < CH3CH2O
Q.2 Which acid of each pair shown here would you expect to be stronger?
CH3CO2H or FCH2CO2H
Ans. 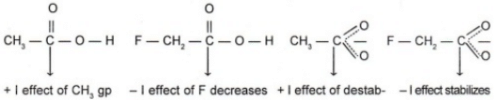
Thus due to lesser electron density in the O — H bond and greater stability of FCH2COO–
ion over CH3COO– ion FCH2COOH is a stronger acid than CH3COOH.
Q.3 Which acid is stronger and why?
Ans.
| F3C - C6H4- COOH, | CH3- C6H4- COOH |
| A. CF3 has a strong (-I) effect. | CH3 has a weak (+1) effect. |
| It stabilizes the carboxylate ion by dispersing the -ve charge. | It stabilizes the carboxylate ion by intensifying the -ve |
Q.4 Arrange the following compounds in increasing order of their reactivity towards HCN. Explain it with proper reasoning. Acetaldehyde, Acetone, Di-tert-butyl ketone, Methyl tert-butyl ketone.
Ans. Addition of HCN to the carboxyl compounds is a nucleophilic addition reaction. The reactivity towards HCN addition decreases as the + I effect of the alkyl groups increases and/or the steric hindrance to the nucleophilic attack by CN– at the carboxyl carbon increases. Thus the reactivity decreases in the order.
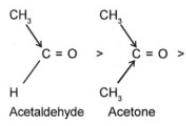
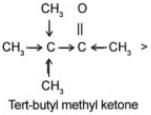
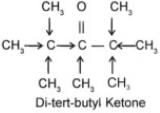
+ I effect increases
Steric hindrance increases.
Reactivity towards HCN addition decreases.
In other words, reactivity increases in the reverse order, i.e. Ditert-butyl Ketone < tert-Butyl methyl Ketone < Acetone < Acetaldehyde
Q.5 Explain why o-hydroxybenzaldehyde is a liquid at room temperature while phydroxybenzaldehyde is a high melting solid.
Ans. Due to interamolecular H-bonding ortho-hydroxy benzaldehyde exists as discrete
molecule whereas due to intermolecular H-bonding, p- hydroxybenzaldehyde exists as
associated molecules. To break these intermolecular H-bonds, a large amount of energy is
needed. Consequently, p-hydroxybenzaldehyde has a much higher m.p. and b.p. than
that of o-hydroxy benzaldehyde. As a result, o-hydroxy benzaldehyde is a liquid at room
temperature while p-hydroxy benzaldehyde is a high melting solid.
Long Answer Type Questions
Q.1 Arrange the following compounds in order of their property as indicated.
Ans.
i. Acetaldehyde, Acetone, di-tert-butyl ketone, Methyl tert-butyl ketone reactivity
towards HCN
- di-tert-butyl ketone < Methyl tert-butyl ketone <Acetone <Acetaldehyde
- aldehydes are more reactive towards nucleophilic addition across the >C=O due to steric and electronic reasons.
- Sterically the presence of two relatively large substituents inketones hinders the approach of nucleophile to carbonyl carbon than in aldehydes having only one such substituent.
- Electronically , the presence of two alkyl groups reduces the electrophilicity of the carbonyl carbon in ketones.
ii. CH3CH2CHBrCOOH, CH3CHBrCH2COOH, (CH3 )2CHCOOH, CH3CH2CH2COOH acid strength
- (CH3 )2CHCOOH <CH3CH2CH2COOH<CH3CHBrCH2COOH< CH3CH2CHBrCOOH
- Electron withdrawing groups like –Br increases the acidity of carboxylic aids by stabilizing the conjugate base through delocalisation of negative charge by negative inductive effect. The closer the electron withdrawing group to the – COOH group, greater is the stabilising effect.
- Electron-donating groups decrease the acidity by destabilizing the conjugate base.greater the number of –CH3 groups, the greater the destabilizing effect and lower the acidity.
iii. Benzoic acid, 4-Nitrobenzoic acid, 3,4-Dinitrobenzoic acid, 4- Methoxybenzoic acid (
acid strength)
As we have seen in the previous case, electron-donating groups decrease the strengths of acids, while electron-withdrawing groups increase the strengths of acids. As methoxy group is an electron-donating group, 4-methoxybenzoic acid is a weaker acid than benzoic acid. Nitro group is an electron-withdrawing group and will increase the strengths of acids. As 3,4-dinitrobenzoic acid contains two nitro groups, it is a slightly stronger acid than 4-nitrobenzoic acid.
Hence, the strengths of the given acids increase as:
4-methoxy benzoic acid < benzoic acid < 4-nitrobenzoic acid< 3,4-dinitrobenzoic acid.
|
361 videos|822 docs|301 tests
|
FAQs on Short & Long Answer Questions: Aldehydes, Ketones & Carboxylic Acids - Chemistry for JEE Main & Advanced
| 1. What are aldehydes, ketones, and carboxylic acids? |  |
| 2. How are aldehydes, ketones, and carboxylic acids named? |  |
| 3. What are some common uses of aldehydes, ketones, and carboxylic acids? |  |
| 4. What are the key differences between aldehydes and ketones? |  |
| 5. How do aldehydes, ketones, and carboxylic acids react? |  |






















