How to Determine Limiting Reagent - NEET PDF Download
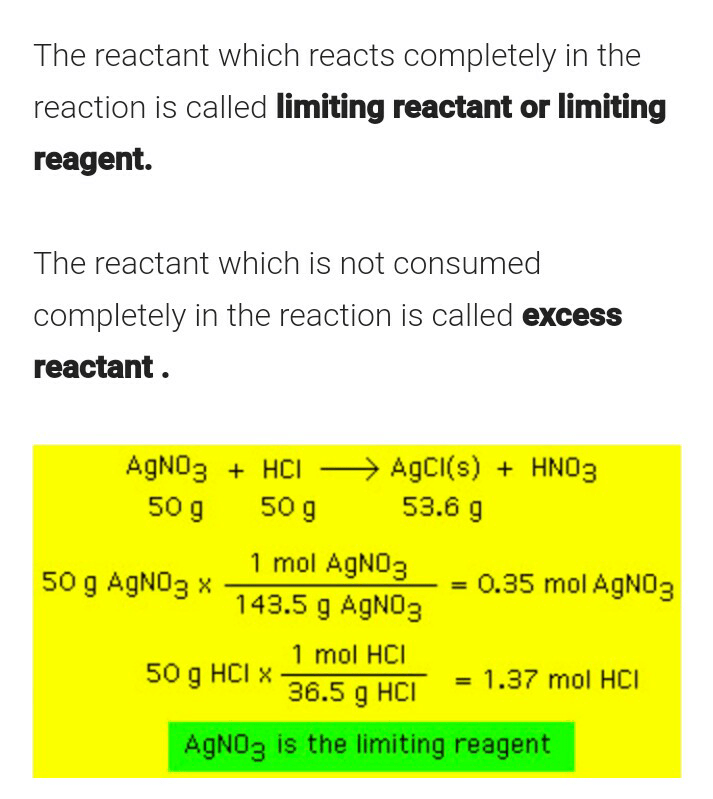
What is Limiting Reagent?
The reactant that is entirely used up in a reaction is called as limiting reagent. This reactant generally determines when the reaction will stop. The exact amount of reactant which will be needed to react with another element can be calculated from the reaction stoichiometry.
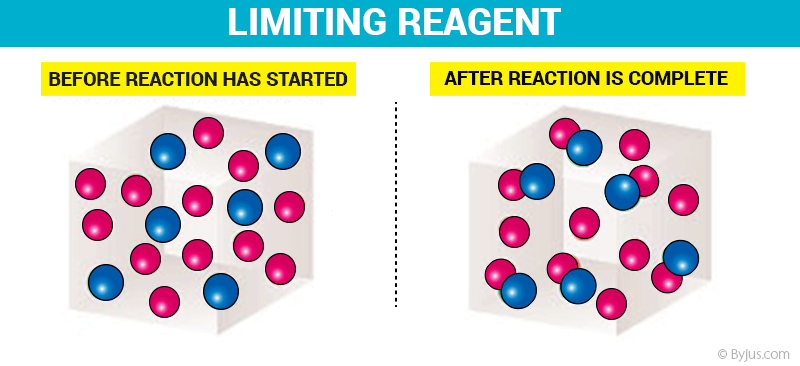
From the illustration shown above, it can be observed that the limiting reactant is the reason the reaction cannot continue since there is nothing left to react with the excess reactant. it is the reactant that entirely consumed over the course of the reaction.
Example
Given: 1 mol of oxygen and 1 mol of hydrogen are present to undergo the following reaction.
2H2 O2 → 2H2O
Since the reaction uses up hydrogen twice as fast as oxygen, the limiting reactant would be hydrogen.
Finding the Limiting Reagent in a Reaction
The Following points should be considered while attempting to identify the limiting reagent:
- When there are only two reactants, write the balanced chemical equation and check the amount of reactant B required to react with reactant A. When the amount of reactant B is greater, the reactant A is the limiting reagent.
- The reactant which is in a lesser amount than is required by stoichiometry is the limiting reactant.
- In an alternate method of finding the limiting agent, the amount of product formed by each reactant is calculated. The limiting reactant is the reactant from which the minimum amount of product is formed. Also if we calculate the amount of one reactant needed to react with another reactant, then the reactant which is in shortage would be the required limiting reactant.
Thus the required limiting reagent for the reaction can be identified using the points provided above. These reagents are very important while calculating the percentage yield of a given reaction.

FAQs on How to Determine Limiting Reagent - NEET
| 1. What is a limiting reagent in a chemical reaction? |  |
| 2. How can I determine the limiting reagent in a chemical reaction? |  |
| 3. Can there be more than one limiting reagent in a chemical reaction? |  |
| 4. What happens if the reactants are not in the stoichiometric ratio? |  |
| 5. How does the limiting reagent affect the yield of a reaction? |  |














