Imperfections in Solids | Physical Chemistry for NEET PDF Download
Stoichiometric Defects
The compounds in which the number of positive and negative ions are exactly in the ratios indicated by their chemical formulae are called stoichiometric compounds. The defects do not disturb the stoichiometry (the ratio of numbers of positive and negative ions) are called stoichiometric defects. These are of following types,
(a) Interstitial defect: This type of defect is caused due to the presence of ions in the normally vacant interstitial sites in the crystals.
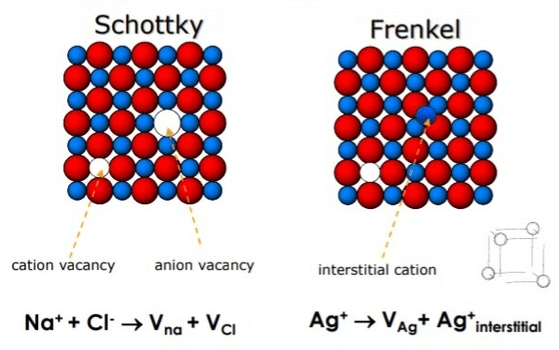
(b) Schottky defect: This type of defect when equal number of cations and anions are missing from their lattice sites so that the electrical neutrality is maintained. This type of defect occurs in highly ionic compounds which have high co-ordination number and cations and anions of similar sizes.
Example: NaCl, KCl, CsCl and KBr etc.
(c) Frenkel defect: This type of defect arises when an ion is missing from its lattice site and occupies an interstitial position. The crystal as a whole remains electrically neutral because the number of anions and cations remain same. Since cations are usually smaller than anions, they occupy interstitial sites. This type of defect occurs in the compounds which have low co-ordination number and cations and anions of different sizes.
Example: ZnS, AgCl and AgI etc.
Frenkel defect are not found in pure alkali metal halides because the cations due to larger size cannot get into the interstitial sites. In AgBr both Schottky and Frenkel defects occur simultaneously.
Non-Stoichiometric Defects:
Nonstoichiometric inorganic solids contain the constituent elements in a non-stoichiometric ratio due to defects in their crystal structures.
These defects are of two types:
(i) metal excess defect and
(ii) metal deficiency defect.
(i) Metal Excess Defect.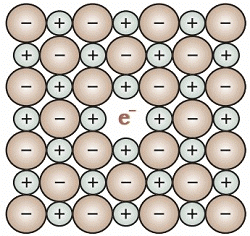
⇒ Metal excess defect due to anionic vacancies.
⇒ This type of defect is exhibited by alkali halides like NaCl and KCl.
⇒ Application of heat to NaCl in an atmosphere of sodium vapour results in deposition of the sodium atoms on the surface of the crystal.
⇒ The sodium atoms lose electron to form Na+.
⇒ The Cl– ions diffuse to the surface of the crystal and combine with Na atoms to give NaCl.
⇒ The released electrons diffuse into the crystal and occupy anionic sites.
⇒ The anionic sites occupied by unpaired electrons are called F-centres that imparts yellow colour to the crystals of NaCl due to the excitation of the electrons on absorption of energy from the visible light falling on the crystals.
⇒ Excess of lithium makes LiCl crystals pink and excess of potassium makes KCl crystals violet.
⇒ Metal excess defect due to the presence of extra cations at interstitial sites:
⇒ Heating Zinc oxide that exists in white colour at room temperature loses oxygen and turns yellow.
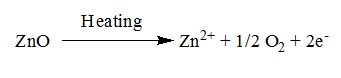 ⇒ This results in excess of zinc in the crystal and its formula becomes Zn1+xO.
⇒ This results in excess of zinc in the crystal and its formula becomes Zn1+xO.
⇒ The excess Zn2+ ions move to interstitial sites and the electrons to neighbouring interstitial sites.
Metal Deficiency Defect:
⇒ Many solids possess less amount of the metal as compared to the stoichiometric proportion.
⇒ For example, FeO is mostly found with a composition ranging from Fe93O to Fe0.96O.
⇒ In crystals of FeO some Fe2+ cations are missing and the loss of positive charge is made up by the presence of required number of Fe3+.
|
117 videos|226 docs|237 tests
|
FAQs on Imperfections in Solids - Physical Chemistry for NEET
| 1. What are some common imperfections in solids? |  |
| 2. How do point defects affect the properties of solids? |  |
| 3. What are dislocations and how do they impact the mechanical properties of solids? |  |
| 4. How do grain boundaries affect the properties of polycrystalline materials? |  |
| 5. How can imperfections in solids be controlled or manipulated in materials engineering? |  |





















