Conductors, Insulators, and Semiconductors | Basic Physics for IIT JAM PDF Download
Materials are classified as conductors, insulators, or semiconductors according to their electric conductivity. The classifications can be understood in atomic terms. Electrons in an atom can have only certain well-defined energies, and, depending on their energies, the electrons are said to occupy particular energy levels. In a typical atom with many electrons, the lower energy levels are filled, each with the number of electrons allowed by a quantum mechanical rule known as the Pauli exclusion principle. Depending on the element, the highest energy level to have electrons may or may not be completely full. If two atoms of some element are brought close enough together so that they interact, the two-atom system has two closely spaced levels for each level of the single atom. If 10 atoms interact, the 10-atom system will have a cluster of 10 levels corresponding to each single level of an individual atom. In a solid, the number of atoms and hence the number of levels is extremely large; most of the higher energy levels overlap in a continuous fashion except for certain energies in which there are no levels at all. Energy regions with levels are called energy bands, and regions that have no levels are referred to as band gaps.
The highest energy band occupied by electrons is the valence band. In a conductor, the valence band is partially filled, and since there are numerous empty levels, the electrons are free to move under the influence of an electric field; thus, in a metal the valence band is also the conduction band. In an insulator, electrons completely fill the valence band; and the gap between it and the next band, which is the conduction band, is large. The electrons cannot move under the influence of an electric field unless they are given enough energy to cross the large energy gap to the conduction band. In a semiconductor, the gap to the conduction band is smaller than in an insulator. At room temperature, the valence band is almost completely filled. A few electrons are missing from the valence band because they have acquired enough thermal energy to cross the band gap to the conduction band; as a result, they can move under the influence of an external electric field. The “holes” left behind in the valence band are mobile charge carriers but behave like positive charge carriers.
For many materials, including metals, resistance to the flow of charge tends to increase with temperature. For example, an increase of 5° C (9° F) increases the resistivity of copper by 2 percent. In contrast, the resistivity of insulators and especially of semiconductors such as silicon and germanium decreases rapidly with temperature; the increased thermal energy causes some of the electrons to populate levels in the conduction band where, influenced by an external electric field, they are free to move. The energy difference between the valence levels and the conduction band has a strong influence on the conductivity of these materials, with a smaller gap resulting in higher conduction at lower temperatures.
The values of electric resistivities listed in Table 2 show an extremely large variation in the capability of different materials to conduct electricity. The principal reason for the large variation is the wide range in the availability and mobility of charge carriers within the materials. The copper wire in Figure 12, for example, has many extremely mobile carriers; each copper atom has approximately one free electron, which is highly mobile because of its small mass. An electrolyte, such as a saltwater solution, is not as good a conductor as copper. The sodium and chlorine ions in the solution provide the charge carriers. The large mass of each sodium and chlorine ion increases as other attracted ions cluster around them. As a result, the sodium and chlorine ions are far more difficult to move than the free electrons in copper. Pure water also is a conductor, although it is a poor one because only a very small fraction of the water molecules are dissociated into ions. The oxygen, nitrogen, and argon gases that make up the atmosphere are somewhat conductive because a few charge carriers form when the gases are ionized by radiation from radioactive elements on Earth as well as from extraterrestrial cosmic rays (i.e., high-speed atomic nuclei and electrons). Electrophoresis is an interesting application based on the mobility of particles suspended in an electrolytic solution. Different particles (proteins, for example) move in the same electric field at different speeds; the difference in speed can be used to separate the contents of the suspension.
A current flowing through a wire heats it. This familiar phenomenon occurs in the heating coils of an electric range or in the hot tungsten filament of an electric light bulb. This ohmic heating is the basis for the fuses used to protect electric circuits and prevent fires; if the current exceeds a certain value, a fuse, which is made of an alloy with a low melting point, melts and interrupts the flow of current. The power P dissipated in a resistance R through which current i flows is given by
P=iR2 (22)
where P is in watts (one watt equals one joule per second), i is in amperes, and R is in ohms. According to Ohm’s law, the potential difference V between the two ends of the resistor is given by V = iR, and so the power P can be expressed equivalently as
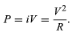 (23)
(23)
In certain materials, however, the power dissipation that manifests itself as heat suddenly disappears if the conductor is cooled to a very low temperature. The disappearance of all resistance is a phenomenon known as superconductivity. As mentioned earlier, electrons acquire some average drift velocity v under the influence of an electric field in a wire. Normally the electrons, subjected to a force because of an electric field, accelerate and progressively acquire greater speed. Their velocity is, however, limited in a wire because they lose some of their acquired energy to the wire in collisions with other electrons and in collisions with atoms in the wire. The lost energy is either transferred to other electrons, which later radiate, or the wire becomes excited with tiny mechanical vibrations referred to as phonons. Both processes heat the material. The term phonon emphasizes the relationship of these vibrations to another mechanical vibration—namely, sound. In a superconductor, a complex quantum mechanical effect prevents these small losses of energy to the medium. The effect involves interactions between electrons and also those between electrons and the rest of the material. It can be visualized by considering the coupling of the electrons in pairs with opposite momenta; the motion of the paired electrons is such that no energy is given up to the medium in inelastic collisions or phonon excitations. One can imagine that an electron about to “collide” with and lose energy to the medium could end up instead colliding with its partner so that they exchange momentum without imparting any to the medium.
A superconducting material widely used in the construction of electromagnets is an alloy of niobium and titanium. This material must be cooled to a few degrees above absolute zero temperature, −263.66° C (or 9.5 K), in order to exhibit the superconducting property. Such cooling requires the use of liquefied helium, which is rather costly. During the late 1980s, materials that exhibit superconducting properties at much higher temperatures were discovered. These temperatures are higher than the −196° C of liquid nitrogen, making it possible to use the latter instead of liquid helium. Since liquid nitrogen is plentiful and cheap, such materials may provide great benefits in a wide variety of applications, ranging from electric power transmission to high-speed computing.
|
213 videos|156 docs|94 tests
|






















