Past Year Questions: Quality & Characteristics of Waste Water | Environmental Engineering - Civil Engineering (CE) PDF Download
Q1: A wastewater sample contains two nitrogen species, namely ammonia and nitrate. Consider the atomic weight of N, H, and O as 14 g/mol, 1 g/mol, and 16 g/mol, respectively. In this wastewater, the concentration of ammonia is 34 mg NH3/liter and that of nitrate is 6.2 mg NO−3/liter. The total nitrogenconcentration in this wastewater is _______ milligram nitrogen per liter. (round off to one decimal place) (2022 SET-1)
Ans: 29 to 30
Sol: Total nitrogen concentration (mg/liter) in this wastewater = 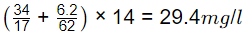 as Nitrogen.
as Nitrogen.
Q2: The total hardness in raw water is 500 milligram per liter as CaCO3. The total hardness of this raw water, expressed in milligram equivalent per liter, is
(Consider the atomic weights of Ca, C, and O as 40 g/mol, 12 g/mol, and 16 g/mol, respectively.) (2022 SET-1)
(a) 10
(b) 100
(c) 1
(d) 5
Ans: (a)
Sol: 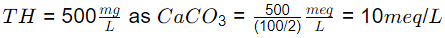
Q1: Ammonia nitrogen is present in a given wastewater sample as the ammonium ion (NH+4) and ammonia (NH3). If pH is the only deciding factor for the proportion of these two constituents, which of the following is a correct statement? (2021 SET-1)
(a) At pH above 9.25, only (NH+4) will be present
(b) At pH above 9.25, (NH3) will be predominant
(c) At pH 7.0, (NH+4) and (NH3) will be found in equal measures
(d) At pH 7.0, (NH+4) will be predominant
Ans: (d)
Sol:
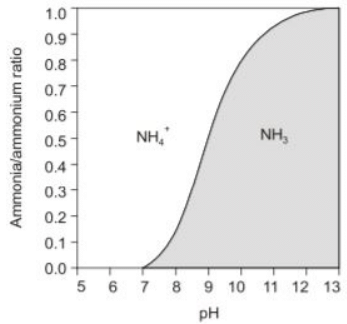
From the above curve, it is evident that at pH 7.0, (NH+4) will be predominant.
Q2: A 50 mL sample of industrial wastewater is taken into a silica crucible. The empty weight of the crucible is 54.352 g. The crucible with the sample is dried in a hot air oven at 104ºC till a constant weight of 55.129 g. Thereafter, the crucible with the dried sample is fired at 600ºC for 1 h in a muffle furnace, and the weight of the crucible along with residue is determined as 54.783 g. The concentration of total volatile solids is (2021 SET-1)
(a) 15540 mg/L
(b) 8620 mg/L
(c) 6920 mg/L
(d) 1700 mg/L
Ans: (c)
Sol: V = 50 ml
W1 = 54.352 gm
W2 = 55.129 gm
W3 = 54.783 gm
Total volatile solids,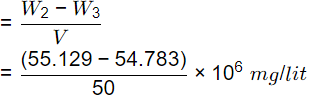
= 6920 mg/l
Q1: A completely mixed dilute suspension of sand particles having diameters 0.25, 0.35, 0.40, 0.45 and 0.50 mm are filled in a transparent glass column of diameter 10 cm and height 2.50 m. The suspension is allowed to settle without any disturbance. It is observed that all particles of diameter 0.35 mm settle to the bottom of the column in 30 s. For the same period of 30 s, the percentage removal (round off to integer value) of particles of diameters 0.45 and 0.50 mm from the suspension is ____ (2019 SET-1)
Ans: 100 to 100
Sol: Since sand particle of size 0.35 mm settles to the bottom of the column in 30 sec particles having size greater than 0.35 mm i.e. 0.45 and 0.50 mm will also settle in suspension at the bottom of column by 100% in 30 sec, infact these bigger sized particle will settle by 100% in less than 30 sec. So answer is 100%.
Q1: The ultimate BOD (L0) a wastewater sample is estimated as 87% of COD. The COD of this wastewater is 300 mg/L. Considering first order BOD reaction rate constant k (use natural log) = 0.23 per day and temperature coefficient θ = 1.047, the BOD value (in mg/L, up to one decimal place) after three days of incubation at 2 7 °C for the w astew ater will b e _______ [2018 : 2 Marks, Set-I]
Ans: 160.226 mg/l
Sol: Ultimate BOD = 0.87
COD = 0.87 x 300 = 261 mg/l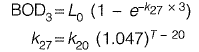
For municipal sewage, at standard temperature, value of k((base e) = 0.23 per day. Thus, value 0.23 per day given is w.r.t. the standard temperature of 20°C.
= 0.23 (1,047)27-20
= 0.317 days-1
BOD3 = 261 (1 - e-0.317 x 3)
= 160.226 mg/l
Q1: For a given water sample, the ratio between BOD5-day, 2o°c and the ultimate BOD is 0.68. The value of the reaction rate constant k (on base e) (in day-1, up to two decimal places ) is [2017 : 2 Marks, Set-II]
Ans: 0.23 day-1
Sol:
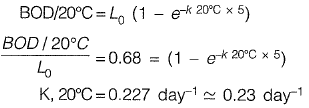
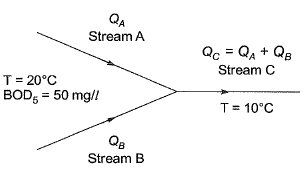
Q1: Two wastewater streams A and B, having an indentical ultimate BOD are getting mixed to form the stream C. The temperature of the stream /A is 20°C and the temperature of the stream C is 10°C. It is given that
- the 5-day BOD of the stream A measured at 20°C = 50 mg/l
- BOD rate constant (base 10) at 20°C = 0.115 per day
- temperature coefficient = 1.135
The 5-day BOD (in mg/l, up to one decimal place) of the stream C, calculated at 10°C , is [2017 : 2 Marks, Set-I]
Ans: 21.21mg/l
Sol:
For stream A,
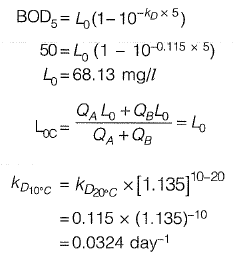
For stream C,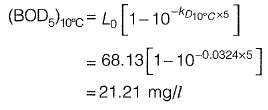
Q1: For a wastewater sample, the three-day biochemical oxygen demand at incubation temperature of 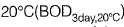 is estimated as 200 mg/l. Taking the value of the first order BOD reaction rate constant as 0.22 day-1, the five-day BOD (expressed in mg/l) of the wastewater at incubation temperature of
is estimated as 200 mg/l. Taking the value of the first order BOD reaction rate constant as 0.22 day-1, the five-day BOD (expressed in mg/l) of the wastewater at incubation temperature of 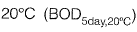 would be______ . [2016 : 2 Marks, Set-Il]
would be______ . [2016 : 2 Marks, Set-Il]
Ans: 276.19 mg/l
Sol:
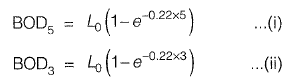
From equation (i) and (ii), we get,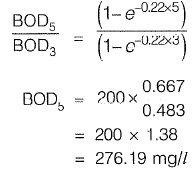
Q2: The 2-day and 4-day BOD values of a sewage sample are 100 mg/l and 155 mg/l, respectively. The value of BOD rate constant (expressed in per day) is________ . [2016 : 2 Marks, Set-I]
Ans: 0.299
Sol:
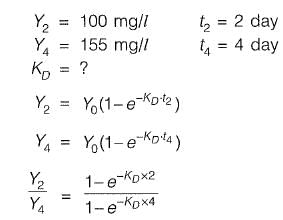
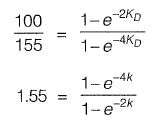
1.55 - 1,55x = 1 - x2
x2 - 1.55x + 0.55= 0
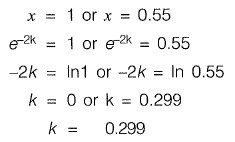
Q1: Ultimate BOD of a river water sample is 20 mg/L. BOD rate constant (natural log) is 0.15 day-1. The respective values of BOD (in %) exerted and remaining after 7 days are: [2015 : 2 Marks, Set-II]
(a) 45 and 55
(b) 55 and 45
(c) 65 and 35
(d) 75 and 25
Ans: (c)
Sol: BOD remaining after 7 days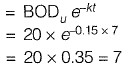
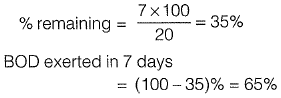
|
14 videos|120 docs|98 tests
|
FAQs on Past Year Questions: Quality & Characteristics of Waste Water - Environmental Engineering - Civil Engineering (CE)
| 1. What are the main characteristics of wastewater? |  |
| 2. How does wastewater affect the environment? |  |
| 3. What are the different types of wastewater? |  |
| 4. What is the significance of treating wastewater? |  |
| 5. What are common methods used in wastewater treatment? |  |





















