NCERT Exemplar: Structure of the Atom | Science Class 9 PDF Download
Multiple Choice Questions
Q.1. Which of the following correctly represent the electronic distribution in the Mg atom?
(a) 3, 8, 1
(b) 2, 8, 2
(c) 1, 8, 3
(d) 8, 2, 2
Ans: (b)
Explanation: Atomic number of Mg is 12 hence electronic distribution will be 1s22s22p63s2.
Q.2. Rutherford’s ‘alpha (α) particles scattering experiment’ resulted in to discovery of
(a) Electron (b) Proton
(c) Nucleus in the atom
(d) Atomic mass
Ans: (c)
Explanation: Rutherford’s ‘alpha (α) particles scattering experiment’ experiment concludes that alpha particles returned to their original path. This showed the presence of a nucleus in the centre.
Q.3. The number of electrons in an element X is 15 and the number of neutrons is 16. Which of the following is the correct representation of the element?
(a) 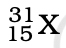
(b)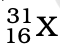
(c) 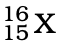
(d) 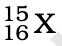
Ans: (a)
Explanation: Number of protons in an element depicts atomic number. A number of protons and electrons are equal in an element. Hence atomic number is written in subscript whereas the mass number is written in the subscript before the symbol of the element.
Q.4. Dalton’s atomic theory successfully explained
(i) Law of conservation of mass
(ii) Law of constant composition
(iii) Law of radioactivity
(iv) Law of multiple proportion
(a) (i), (ii) and (iii)
(b) (i), (iii) and (iv)
(c) (ii), (iii) and (iv)
(d) (i), (ii) and (iv)
Ans: (d)
Explanation: Dalton’s theory explains Law of conservation of mass, Law of constant composition, Law of multiple proportions. But it never gives any details of Law of radioactivity.
Q.5. Which of the following statements about Rutherford’s model of atom are correct?
(i) considered the nucleus as positively charged
(ii) established that the α–particles are four times as heavy as a hydrogen atom
(iii) can be compared to solar system
(iv) was in agreement with Thomson’s model
(a) (i) and (iii)
(b) (ii) and (iii)
(c) (i) and (iv)
(d) only (i)
Ans: (a)
Explanation: Positively charged alpha particles were deflected by the nucleus. This shows the nucleus is positively charged. Rutherford also postulated that electrons are arranged in an atom around the nucleus like planets arranged around the sun.
Q.6. Which of the following are true for an element?
(i) Atomic number = number of protons + number of electrons
(ii) Mass number = number of protons + number of neutrons
(iii) Atomic mass = number of protons = number of neutrons
(iv) Atomic number = number of protons = number of electrons
(a) (i) and (ii)
(b) (i) and (iii)
(c) (ii) and (iii)
(d) (ii) and (iv)
Ans: (d)
Explanation: Atomic number Z is the number of protons present in an electron which is also equal to a number of the electron in an atom. Since the mass of a neutron is negligible, a number of protons and electron is added to obtain a mass number of an element.
Q.7. In the Thomson’s model of atom, which of the following statements are correct?
(i) the mass of the atom is assumed to be uniformly distributed over the atom
(ii) the positive charge is assumed to be uniformly distributed over the atom
(iii) the electrons are uniformly distributed in the positively charged sphere
(iv) the electrons attract each other to stabilise the atom
(a) (i), (ii) and (iii)
(b) (i) and (iii)
(c) (i) and (iv)
(d) (i), (iii) and (iv)
Ans: (a)
Explanation: Thomson’s model postulated that the atom’s mass and positive charge were uniformly distributed, and the electrons were embedded in it like plums in a pudding. Option (iv) is incorrect, as electrons do not attract each other in this model. Hence, (i), (ii), and (iii) are correct.
Q.8. Rutherford’s α–particle scattering experiment showed that
(i) electrons have negative charge
(ii) the mass and positive charge of the atom is concentrated in the nucleus
(iii) neutron exists in the nucleus
(iv) most of the space in atom is empty Which of the above statements are correct?
(a) (i) and (iii)
(b) (ii) and (iv)
(c) (i) and (iv)
(d) (iii) and (iv)
Ans: (b)
Explanation: An atom consists of a positively charged, dense and very small nucleus which have all the protons and neutrons. The positive charge is due to protons, as neutrons have no charge. Here the space is empty because alpha particles pass straight through the gold foil without any deflection. Thomson explained that electron s have a negative charge. Existence of neutron was discovered by Chadwick.
Q.9. The ion of an element has 3 positive charges. Mass number of the atom is 27 and the number of neutrons is 14. What is the number of electrons in the ion?
(a) 13
(b) 10
(c) 14
(d) 16
Ans: (b)
Explanation:
Mass number (A) of the atom = 27
Number of the neutron in the atom =14
Number of Electrons = Mass number-Number of neutrons = 27-14
Number of electrons= 13
Since ions of the element have 3 positive charges number of the electron in the ion is 13-3 which equal 10.
Hence the answer is 10
Q.10. Identify the Mg2+ ion from the Fig.4.1 where, n and p represent the number of neutrons and protons respectively.
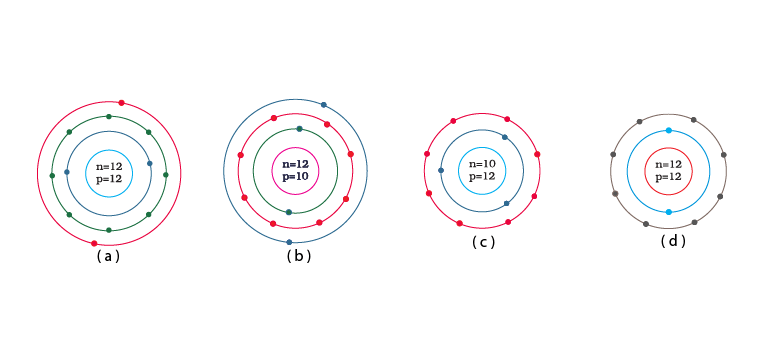 Explanation: Number of protons in Mg atom = 2+ 8 + 2 = 12
Explanation: Number of protons in Mg atom = 2+ 8 + 2 = 12
Number of neutrons in Mg atom = 24 -12 = 12
[as mass number of Mg atom = 24 and number of neutrons = mass number – number of protons]
Therefore, option (d) is the correct answer
Q.11. In a sample of ethyl ethanoate (CH3COOC2H5) the two oxygen atoms have the same number of electrons but different number of neutrons. Which of the following is the correct reason for it?
(a) One of the oxygen atoms has gained electrons
(b) One of the oxygen atoms has gained two neutrons
(c) The two oxygen atoms are isotopes
(d) The two oxygen atoms are isobars.
Ans: (c)
Explanation: Two Oxygen atoms in CH3COOC2H5 can have a different number of neutrons only if the two O-atoms are isotopes. Isotopes of an element have the same number of protons (and electrons) but a different number of neutrons.
Q.12. Elements with valency 1 are
(a) always metals
(b) always metalloids
(c) either metals or non-metals
(d) always non-metals
Ans: (c)
Explanation: If the element shows positive valency it is a metal and if the element shows negative valency it will be a non-metal.
Q.13. The first model of an atom was given by
(a) N. Bohr
(b) E. Goldstein
(c) Rutherford
(d) J.J. Thomson
Ans: (d)
Explanation: J.J. Thomson proposed the Plum Pudding Model in 1897. He described the atom as a sphere of positive charge with negatively charged electrons embedded in it, like plums in a pudding.
Q.14. An atom with 3 protons and 4 neutrons will have a valency of
(a) 3
(b) 7
(c) 1
(d) 4
Ans: (c)
Explanation: Atomic number = 3 (equal to the number of protons) → Lithium (Li). Electronic configuration = 2, 1. To achieve a stable configuration (like noble gases), Lithium loses 1 electron, making its valency = 1.
Q.15. The electron distribution in an aluminium atom is
(a) 2, 8, 3
(b) 2, 8, 2
(c) 8, 2, 3
(d) 2, 3, 8
Ans: (a)
Explanation: Atomic number of Aluminium is 13, the First shell can have a maximum of 2 electron and the second shell holds a maximum of 8 electrons. Hence option a is the right answer.
Q.16. Which of the following in Fig. 4.2 do not represent Bohr’s model of an atom correctly?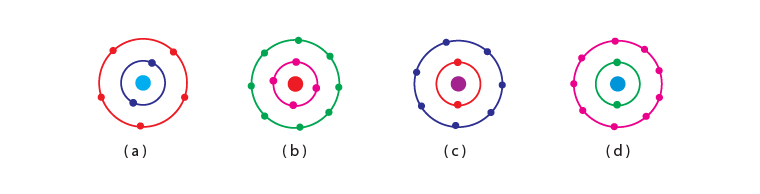 (a) (i) and (ii)
(a) (i) and (ii)
(b) (ii) and (iii)
(c) (ii) and (iv)
(d) (i) and (iv)
Ans: (c)
Explanation: The first shell can have a maximum of 2 electron and the second shell can have a maximum of 8 electrons hence ii and iv do not represent Bohr’s model of an atom correctly.
Q.17. Which of the following statement is always correct?
(a) An atom has equal number of electrons and protons.
(b) An atom has equal number of electrons and neutrons.
(c) An atom has equal number of protons and neutrons.
(d) An atom has equal number of electrons, protons and neutrons.
Ans: (a)
Explanation: An atom is electrically neutral because the number of protons is always equal to a number of electrons. Hence option a) is right.
Q.18. Atomic models have been improved over the years. Arrange the following atomic models in the order of their chronological order
(i) Rutherford’s atomic model
(ii) Thomson’s atomic model
(iii) Bohr’s atomic model
(a) (i), (ii) and (iii)
(b) (ii), (iii) and (i)
(c) (ii), (i) and (iii)
(d) (iii), (ii) and (i)
Ans: (c)
Explanation: Thomson proposed the model in 1904, Rutherford in 1911, and Bohr in 1913, making the correct chronological order (ii), (i), and (iii).
Short Answer Questions
Q.19. Is it possible for the atom of an element to have one electron, one proton and no neutron. If so, name the element.
Ans: Yes, Hydrogen is the element which is having only 1 proton and 1 electron and no neutron hence there is no repulsive force in the nucleus hence it is stable.
Q.20. Write any two observations which support the fact that atoms are divisible
Ans: Atoms are divisible based on the following observations:
- The formation of ionic compounds occurs due to the transfer of electrons, indicating that atoms can break down into smaller charged particles.
- Atoms exist in different forms called isotopes, which have varying mass numbers, showing that atoms consist of different particles like electrons, protons, and neutrons.
Q.21. Will 35Cl and 37Cl have different valencies? Justify your answer.
Ans: 35Cl and 37Cl cannot have different valencies because they are the isotopes of the same element.
Q.22. Why did Rutherford select a gold foil in his α–ray scattering experiment?
Ans: Rutherford chose gold foil for his alpha-ray scattering experiment due to its unique properties:
- High malleability: Gold can be made extremely thin, allowing for a minimal layer.
- Thin layer: The gold foil used was about 1000 atoms thick, which was essential for the experiment.
This choice was crucial for observing the behaviour of alpha particles as they interacted with the gold atoms.
Q.23. Find out the valency of the atoms represented by the Fig. 4.3 (a) and (b).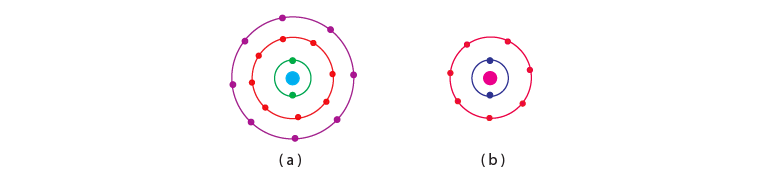
Ans: Atom (a) has zero valencies as it has 8 electrons in its valence shell making the configuration stable. Atom (b) has a valency of +1 as it has 7 electrons in its outermost shell. It can accept 1 electron to achieve octet configuration.
Q.24. One electron is present in the outer most shell of the atom of an element X. What would be the nature and value of charge on the ion formed if this electron is removed from the outer most shell?
Ans: If an electron is removed from the outermost shell a cation will be formed and the charge of the element will be +1.
Q.25. Write down the electron distribution of chlorine atom. How many electrons are there in the L shell? (Atomic number of chlorine is 17).
Ans:
Atomic number of chlorine atom = 17
So, its electronic configuration is
K, L, M
2, 8, 7
L shell of chlorine contains 8 electrons.
Q.26. In the atom of an element X, 6 electrons are present in the outermost shell. If it acquires noble gas configuration by accepting a requisite number of electrons, then what would be the charge on the ion so formed?
Ans: In this atom, 6 electrons are already present in its outermost orbitals. In order to attain a noble gas configuration element has to accept two-electron hence its charge is -2.
Q.27. What information do you get from the Fig. 4.4 about the atomic number, mass number and valency of atoms X, Y and Z? Give your answer in a tabular form.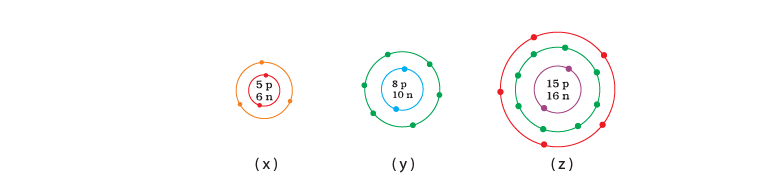 Ans: Atomic number, mass number and valency of atoms X, Y and Z
Ans: Atomic number, mass number and valency of atoms X, Y and Z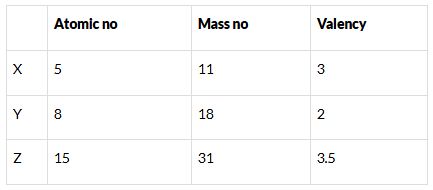
Q.28. In response to a question, a student stated that in an atom, the number of protons is greater than the number of neutrons, which in turn is greater than the number of electrons. Do you agree with the statement? Justify your answer.
Ans: The statement is wrong because the number of protons can never be greater than the number of neutrons. The number of protons will always be less than or equal to a number of neutrons. The number of electrons and protons are always equal in a neutral atom.
Q.29. Calculate the number of neutrons present in the nucleus of an element X which is represented as
Ans: Mass number = No. of protons + No. of neutrons = 31
∴ Number of neutrons = 31– number of protons
= 31–15
= 16
Thus, element X has 16 neutrons in its nucleus.
Q.30. Match the names of the Scientists given in column A with their contributions towards the understanding of the atomic structure as given in column B
(A) – (B)
(a) Ernest Rutherford – (i) Indivisibility of atoms
(b) J.J.Thomson – (ii) Stationary orbits
(c) Dalton – (iii) Concept of the nucleus
(d) Neils Bohr – (iv) Discovery of electrons
(e) James Chadwick -(v) Atomic number
(f) E. Goldstein – (vi) Neutron
(g) Mosley – (vii) Canal rays
Ans:
(A) – (B)
(a) Ernest Rutherford – (iii) Concept of the nucleus
(b) J.J.Thomson – (iv) Discovery of electrons
(c) Dalton – (i) Indivisibility of atoms
(d) Neils Bohr – (ii) Stationary orbits
(e) James Chadwick – (vi) Neutron
(f) E. Goldstein – (vii) Canal rays
(g) Mosley – (v) Atomic number
Q.31. The atomic number of calcium and argon are 20 and 18 respectively, but the mass number of both these elements is 40. What is the name given to such a pair of elements?
Ans: Elements with different atomic numbers but same mass numbers are known as isobars. Calcium and argon are isobars.
Q.32. Complete Table 4.1 on the basis of information available in the symbols given below
(a)  (b)
(b) 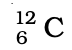 (c)
(c) 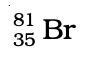
| Element | np | nn |
Ans: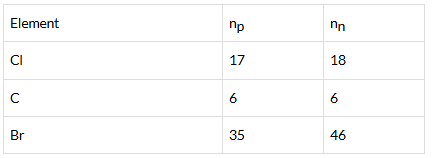
Q.33. Helium atom has 2 electrons in its valence shell but its valency is not 2, Explain
Ans: Helium has 2 electrons in its outermost shell thereby completing duplet configuration. Hence it has no valence shell left empty making its valency 0.
Q.34. Fill in the blanks in the following statements
(a) Rutherford’s α-particle scattering experiment led to the discovery of the __________
(b) Isotopes have same __________but different__________.
(c) Neon and chlorine have atomic numbers 10 and 17 respectively. Their valencies will be__________and__________respectively.
(d) The electronic configuration of silicon is __________and that of sulphur is __________
Ans: (a) Rutherford’s α-particle scattering experiment led to the discovery of the atomic nucleus
(b) Isotopes have same atomic number but different mass number
(c) Neon and chlorine have atomic numbers 10 and 17 respectively. Their valencies will be 0 and 1 respectively.
(d) The electronic configuration of silicon is 2,8,4 and that of sulphur is 2,8,6
Q.35. An element X has a mass number 4 and atomic number 2. Write the valency of this element?
Ans:
Mass number = 4
Atomic number = 2
X is Helium.
It has 0 valency and it will not react with any other atom because it has its outer shell filled.
Long Answer Questions
Q.36. Why do Helium, Neon and Argon have a zero valency?
Ans: Helium has two electrons in its only energy shell, while Argon and Neon have 8 electrons in their valence shells. As these have maximum number of electrons in their valence shells, they do not have any tendency to combine with other elements. Hence, they have a valency equal to zero.
Q.37. The ratio of the radii of hydrogen atom and its nucleus is ~ 105. Assuming the atom and the nucleus to be spherical, (i) what will be the ratio of their sizes? (ii) If atom is represented by planet earth ‘Re’ = 6.4 ×106 m, estimate the size of the nucleus.
Ans: (i) Volume of the sphere = 4/3πr3
Let R be the radius of the atom and r be that of the nucleus.
⇒ R = 105 r
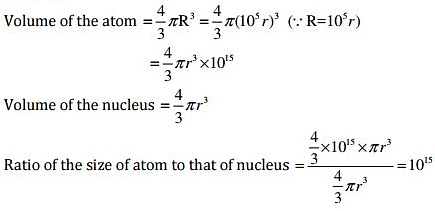
(ii) If the atom is represented by the planet earth (Re = 6.4×106m) then the radius of the nucleus would be rn = Re / 105
rn = 6.4 x 106 m / 105
= 6.5 x 10 m
= 64 m
Q.38. Enlist the conclusions drawn by Rutherford from his α-ray scattering experiment.
Ans: Rutherford concluded from the α-particle scattering experiment that:
- Most of the space inside the atom is empty, as most α-particles passed through the gold foil without deflection.
- Only a few particles were deflected, suggesting that the positive charge of the atom occupies very little space.
- A small fraction of α-particles were deflected by 180°, indicating that all the positive charge and mass of the gold atom are concentrated in a very small volume, known as the nucleus.
From his data, he calculated that the radius of the nucleus is about 100,000 times smaller than that of the atom.
Q.39. In what way is the Rutherford’s atomic model different from that of Thomson’s atomic model?
Ans: Rutherford proposed a model in which electrons revolve around the nucleus in well-defined orbits. There is a positively charged centre in an atom called the nucleus. He also proposed that the size of the nucleus is very small as compared to the size of the atom and nearly all the mass of an atom is centred in the nucleus. Whereas, Thomson proposed the model of an atom to be similar to a christmas pudding. The electrons are studded like currants in a positively charged sphere like christmas pudding and the mass of the atom was supposed to be uniformly distributed.
Q.40. What were the drawbacks of Rutherford’s model of an atom?
Ans: The orbital revolution of the electron is not expected to be stable. Any particle in a circular orbit would undergo a acceleration and the charged particles would radiate energy. Thus, the revolving electron would lose energy and finally fall into the nucleus. If this were so, the atom should be highly unstable and hence matter would not exist in the form that we know.
Q.41. What are the postulates of Bohr’s model of an atom?
Ans: The postulates put forth by Neils Bohr’s about the model of an atom:
(i) Only certain special orbits known as discrete orbits of electrons, are allowed inside the atom
(ii) While revolving in discrete orbits the electrons do not radiate energy.
These orbits are called energy levels.
Energy levels in an atom are shown by circles.
These orbits are represented by the letters K,L,M,N,… or the numbers, n=1,2,3,4,….
Q.42. Show diagramatically the electron distributions in a sodium atom and a sodium ion and also give their atomic number.
Ans:
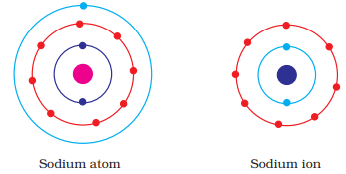
Since the atomic number of sodium atom is 11, it has 11 electrons. A positively charged sodium ion (Na+) is formed by the removal of one electron from a sodium atom. So, a sodium ion has 11 - 1 = 10 electrons in it. Thus, electronic distribution of sodium ion will be 2, 8. The atomic number of an element is equal to the number of protons in its atom. Since, sodium atom and sodium ion contain the same number of protons, therefore, the atomic number of both is 11.
Q.43. In the Gold foil experiment of Geiger and Marsden, that paved the way for Rutherford’s model of an atom, ~ 1.00% of the α-particles were found to deflect at angles > 50º. If one mole of α-particles were bombarded on the gold foil, compute the number of α-particles that would deflect at angles less than 500.
Ans: Total number of α particles used for bombardment = 1 mole
1 mole = 6.022 × 1023 particle
number of α particles deflected at angles greater than 50° (>50°) = 1%
Number of α particles deflected at angles greater than 50° = 100 - 1 = 99%
The actual number of α particles deflected at angles less than 50° = 99 / 100 × 6.022 × 1023
= 596.178 / 100 x 1023
= 5.96 × 1023
|
84 videos|478 docs|60 tests
|
FAQs on NCERT Exemplar: Structure of the Atom - Science Class 9
| 1. What is the structure of an atom and its main components? |  |
| 2. How do the number of protons, neutrons, and electrons determine the identity of an element? |  |
| 3. What is the significance of the atomic mass and how is it calculated? |  |
| 4. What are electron shells, and how do they influence an atom's chemical properties? |  |
| 5. What are isotopes, and why are they important in chemistry and nuclear science? |  |

















