Class 9 Science Chapter 1 Previous Year Questions - Matter in Our Surroundings
SHORT ANSWER TYPE QUESTIONS
Q1:
(a) Convert the following in Celsius:
(i) 373 K
(ii) 400 K
(b) Write three features of solid state of matter.
(c) Explain in brief any three factors that affect the rate of evaporation. [CBSE 2025]
Ans.
(a)
(i) 373 - 273 = 100°C
(ii) 400 - 273 = 127°C
(b)
(i) Fixed shape
(iii) Cannot be compressed much.
(c)
(i) Temperature
(ii) Surface area
(iii) Wind speed.
Q2: Convert 293 K into Celsius scale. [CBSE 2024]
Ans. 293 - 273 = 20ºC
Q3: What is dry ice? [CBSE 2023]
Ans. Solid CO2 is called dry ice.
Q4:
(a) Define matter and write its three states.
(b) Explain how these states of matter arise due to variation in the characteristics of the particles. [CBSE 2023]
Ans.
(a) Matter is substance which has mass and occupies space. Solid, liquid and gaseous are three states of matter.
(b) Solids have fixed shape and volume due to strong forces of attraction between particles and least space between particles.
Liquid state have fixed volume and but not fixed shape due to less force of attraction and more intermolecular space.
Gaseous state do not have fixed shape and volume due to least force of attraction and maximum intermolecular space.
Q5: A substance A has high compressibility and can be easily liquefied. It can take the shape of any container. Predict the nature of the substance. Enlist four properties of this state of matter. [CBSE 2023]
Ans. The substance A is a gas because gases have large empty spaces between the particles. It has high compressibility and can be easily liquefied.
Properties of gases:
(i) Gases have no fixed shape and volume.
(ii) Gases can be compressed on applying pressure to give liquids.
(iii) Particles of a gas are moving in all possible directions with all possible speeds.
(iv) Gases show the property of diffusion.
Q6: State the SI unit of temperature. Mention the boiling point of water and average human body temperature in SI unit. [CBSE 2022]
Ans. SI unit of temperature is Kelvin.
Boiling point of water in SI unit = 100 + 273 = 373 K.
Average body temperature in SI unit = 37 + 273 = 310 K.
Q7: Define evaporation. Explain any two factors affecting its rate. [CBSE 2022]
Ans. Evaporation is a surface phenomenon of change of a liquid into vapours at any temperature below its boiling point.
Factors affecting evaporation:
The rate of evaporation increases with
(i) an increase of surface area
(ii) an increase of temperature
(iii) a decrease in humidity
(iv) an increase in speed of wind.
Q8: Describe an activity to show that air contains water vapours. [CBSE 2021]
Ans.
(i) Take a beaker containing ice-cold water in a tumbler.
(ii) Keep it for some time.
(iii) We will see water droplets at the outer surface of the tumbler.
(iv) It shows there are water vapours in the air, on coming in contact with the cold glass of water, vapours lose energy and get converted to liquid state, which we see in the form of water droplets.
Observation: Water droplets are formed on the surface of glass.
Conclusion: Air containing water vapours which get condensed by coming in contact with the cold surface of glass.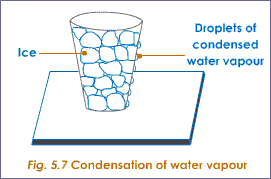 Q9: With the help of an activity show that diffusion becomes faster with the increase in temperature. [CBSE 2021]
Q9: With the help of an activity show that diffusion becomes faster with the increase in temperature. [CBSE 2021]
Ans. Activity/Project:
To study the variation of rate of diffusion with temperature of solid in liquid.
Materials Required: Copper sulphate, two beakers, cold water and hot water.
Procedure:
(i) Take 50 ml of cold water in a beaker.
(ii) Take 50 ml of hot water in another beaker.
(iii) Add a crystal of copper sulphate into the beaker containing 50 ml of cold water.
(iv) Leave them undisturbed.
(v) Record the observations.
Observations: The colour of solution in the first beaker becomes blue slowly whereas the colour becomes blue faster in the second beaker.
Conclusion: The rate of diffusion increases with increase in temperature because kinetic energy of molecules increases.
Q10: Give reasons for the following:
(a) Camphor disappears if kept in open air for a few days.
(b) Wet clothes do not dry easily on a rainy days.
(c) We sweat more on humid days. [CBSE 2020]
Ans.
(a) Camphor has weak inter particle forces of attraction which can be overcome by room temperature and it changes from solid state to vapours directly and disappears.
(b) On rainy days, air is saturated with water vapours, therefore it cannot take more vapours, therefore wet clothes do not dry up easily.
(c) We sweat more on humid days because our sweat does not get evaporate easily due to higher content of water vapours in the atmosphere, therefore, we sweat more and feel uncomfortable in coastal areas where humidity is high.
Q11: Clothes get dry faster in summers than in winters. Give reason. [CBSE2019]
Ans. In summer, the temperature is higher. More water molecules get the higher kinetic energy, leave the surface of clothes and move to the atmosphere. Therefore clothes get dry faster in summer.
Q12: List the three characteristics of particles of matter. [CBSE 2018]
Ans.
(i) Particles of matter have space between them.
(ii) Particles of matter are continuously moving.
(iii) Particles of matter attract each other.
Q13: With the help of an activity show that gases are more easily compressible than liquids and solids. [CBSE 2018]
Ans.
- Take three 10 ml syringes and close their nozzles by rubber corks, as shown in the figure.
- Remove pistons from all the syringes.
- Leaving one syringe, put water in another syringe, chalk pieces in the third syringe.
- Insert pistons back into the syringes.
- Apply some vaseline on the pistons before inserting them into the syringes for their smooth movement.
- Try to compress the content by pushing the piston in each syringe.
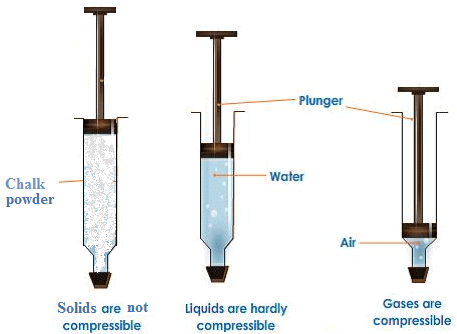 Fig: Gases are highly compressible as compared to solids and liquids
Fig: Gases are highly compressible as compared to solids and liquids
Observations: Piston of first syringe can be moved very easily but less easily in second and is most difficult in third.
Conclusion: Gases can be compressed more easily than liquids and liquids can be compressed more easily than solids.
Q14: Write one similarity between three states of matter. [CBSE 2018]
Ans. All the three states of matter are made up of particles.
Q15: Why mixture does not have a fixed melting or a fixed boiling point? Give two reasons. [CBSE 2017]
Ans.
(a) It does not have fixed composition.
(b) It does not have uniform ordered arrangement of particles.
LONG ANSWER TYPE QUESTIONS
Q1:
(a) How many states does matter have? Write properties of gaseous state.
(b) What type of clothes should we wear in summer? Why? [CBSE 2025]
Ans.
(a) There are three states of matter.
(i) Solid
(ii) Liquid
(iii) Gas
Properties of gaseous state are as follows:
(i) No fixed shape.
(ii) No fixed volume.
(iii) Highly compressible.
(iv) Are fluid.
(b) We should wear cotton clothes. Cotton absorbs sweat more than synthetic clothes. The sweat absorbed by cotton clothes evaporates by the heat of sun and wind. And cooling is produced.
Q2: What is Evaporation? Give the detailed explanation of factors affecting the evaporation. [CBSE 2025]
Ans. The phenomenon of change of liquid into vapour at any temperature below its boiling point is called evaporation.
Factors affecting evaporation:
Effect of temperature: Evaporation increases with the increase of temperature. As the temperature increases, more and more molecules gain energy and leave the surface of the liquid and go into the atmosphere.
Surface area: Evaporation is a surface phenomenon. The number of molecules on the surface of the liquid increases with the surface area. As the number of molecules on the surface of the liquid increases, more evaporation takes place.
Wind velocity: A greater wind velocity takes away more vapours of the liquid in the surroundings. This helps in greater evaporation of more molecules. Thus, evaporation is increased with increase in wind velocity.
Q3: Wet clothes dry up similarly when we spill water on the floor it dries up after some time. In both the cases change of state from liquid to vapour takes place without reaching the boiling point.
(i) What is this phenomenon called?
(ii) Explain how the change occurs at temperatures lower than the boiling point.
(iii) Mention three factors which determine the rate at which the change of state from water to vapours occurs at room temperature. [CBSE 2023]
Ans.
(i) This phenomenon is called evaporation.
(ii) Molecules of water which are moving at speed higher than the average speed leave the surface and go into the atmosphere. This is how evaporation takes place.
(iii) Factors that determine the rate of evaporation:
(а) Surface Area - Greater the surface area, greater will be the rate of evaporation.
(b) Temperature - Higher the temperature, greater will be the rate of evaporation.
Q4:
(a) What is meant by the word ‘Latent’ in latent heat?
(b) Explain with example of water:
(i) latent heat of fusion, and
(ii) latent heat of vaporization [CBSE 2023]
Ans.
(a) The meaning of latent is hidden as it is stored in solid or liquid.
(b) (i) Latent heat of fusion: When ice is melted at 0°C, ice changes into water completely but the temperature remains constant. The energy required to melt one mole of ice into water at 0°C is called latent heat of fusion.
(ii) Water on heating changes into vapours. When temperature reaches 100°C water changes into steam completely but the temperature remains constant. The energy required to convert 1 mole of liquid into vapours completely at its boiling point is called latent heat of vapourization.
Q5:
(a) Convert 574 K to the Celsius scale.
(b) What will be the state of water at:
(i) 108°C
(ii) 275 K
(iii) 370 K
(c) Give reason-why water at room temperature is a liquid? [CBSE 2022]
Ans.
(a)
(i) 574 - 273 = 301°C
(b)
(i) Steam (gas)
(ii) liquid
(iii) liquid.
(c) At room temperature, forces of attraction between molecules of water are not high and the position of particles are not fixed, particles are moving and water can flow from higher level to lower level. It shows water is liquid at room temperature.
Q6:
(a) Define diffusion. Explain the rate of order of diffusion in solids, liquids and gases.
(b) State the effect of temperature on diffusion. [CBSE 2022]
Ans.
(a) Diffusion: The process of intermixing of two or more substances is called diffusion. The rate of diffusion is fastest in gases due to more kinetic energy and less intermolecular forces of attraction.
The rate of diffusion is less in liquids due to less kinetic energy and more force of attraction between particles. The rate of diffusion is minimum in solids due to least kinetic energy and maximum inter particle force of attraction.
(b) The rate of diffusion increases with increase in temperature because kinetic energy of molecules increases.
Q7: Describe an activity to determine the boiling point of water and melting point of ice. [CBSE 2021]
Ans.
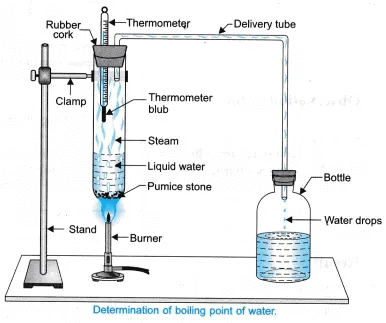
- Take water in a test tube.
- Set the apparatus as shown in diagram.
- Heat water with the help of a burner.
- Note down the reading when temperature of the thermometer becomes constant.
Observation: The temperature becomes constant at 100°C.
Conclusion: The boiling point of water is 100°C
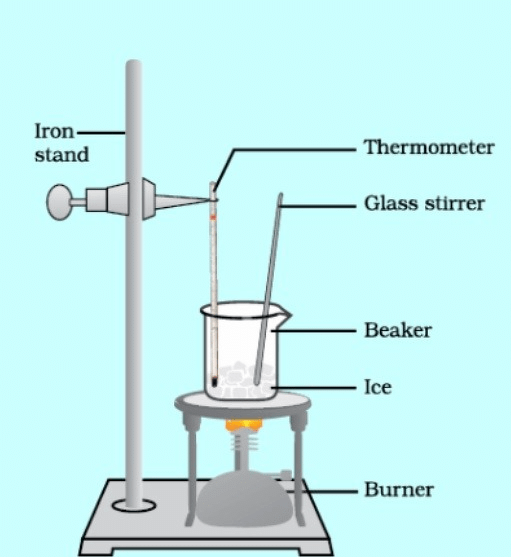
- Take crushed ice in the beaker
- Set the apparatus as shown in diagram.
- Heat the beaker with the help of burner.
- Note down the temperature at which ice changes into liquid and temperature remains constant.
Observation: The temperature becomes constant at 0°C.
Conclusion: The melting point of ice is 0°C.
|
84 videos|478 docs|60 tests
|
FAQs on Class 9 Science Chapter 1 Previous Year Questions - Matter in Our Surroundings
| 1. What are the three states of matter? |  |
| 2. How does a substance change from one state to another? |  |
| 3. What is the difference between evaporation and boiling? |  |
| 4. How does the intermolecular force affect the state of matter? |  |
| 5. Why does a substance expand when heated and contract when cooled? |  |

















