Atoms and Molecules Class 9 Notes Science Chapter 3
| Table of contents |

|
| Introduction |

|
| Laws of Chemical Combination |

|
| What is an Atom? |

|
| What is a Molecule? |

|
| Molecules of Compounds |

|
| Writing Chemical Formulae |

|
| Molecular Mass |

|
Introduction
Atoms and molecules are the building blocks of matter, forming the foundation for everything we see and touch in the world around us. Understanding these fundamental particles helps us grasp how different substances interact and combine to create the diverse materials and phenomena we encounter daily
- Maharishi Kanad and Pakudha Katyayama in India suggested that matter can be divided into smaller particles called Parmanu.
- Democritus and Leucippus in Greece proposed that matter can be divided until it reaches indivisible particles called atoms.
- These ideas were based on philosophical considerations and lacked experimental validation until the eighteenth century.
- In the late 18th century, Antoine L. Lavoisier laid the foundation of chemical sciences by establishing two important laws of chemical combination.
- Lavoisier and Joseph L. Proust conducted numerous experiments to establish the laws of chemical combinations.
- These laws of chemical combination became crucial in understanding the combination and behavior of elements and compounds.
Laws of Chemical Combination
Understanding the fundamental principles that govern chemical reactions is crucial for grasping the nature of matter. Two key laws—the Law of Conservation of Mass and the Law of Constant Proportion—established through the pioneering work of Lavoisier and Proust, provide essential insights into how substances interact and combine."
1. Law of Conservation of Mass
Law of conservation of mass states that mass can neither be created nor destroyed in a chemical reaction.
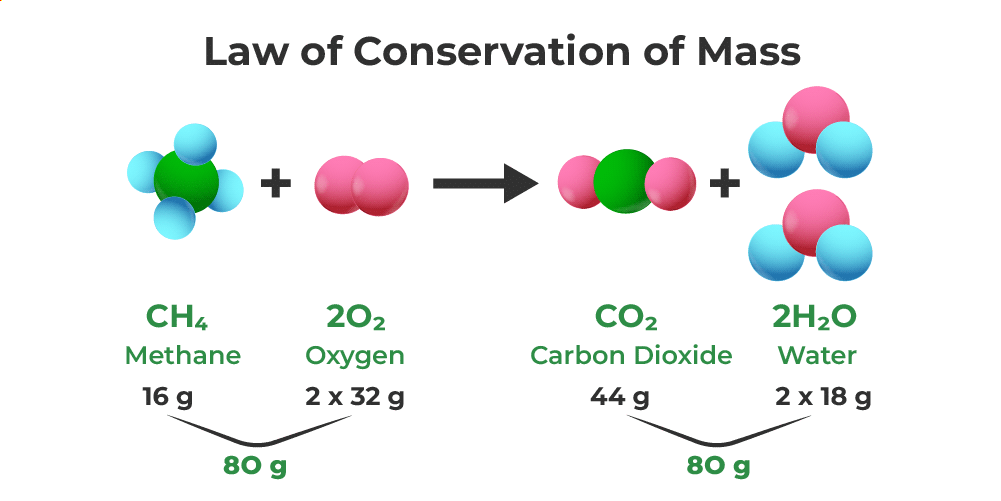 Example of Law of conservation of Mass
Example of Law of conservation of Mass
The Law of Conservation of Mass, established by Lavoisier and Joseph L. Proust, addresses the question of mass change during a chemical reaction. An experiment is conducted using different pairs of chemicals (X and Y), observing the mass before and after mixing the solutions. The law asserts that mass remains constant in a chemical reaction—neither created nor destroyed.
2. Law of Constant Proportion
The law states that in a chemical substance, the elements are always present in definite proportions by mass.
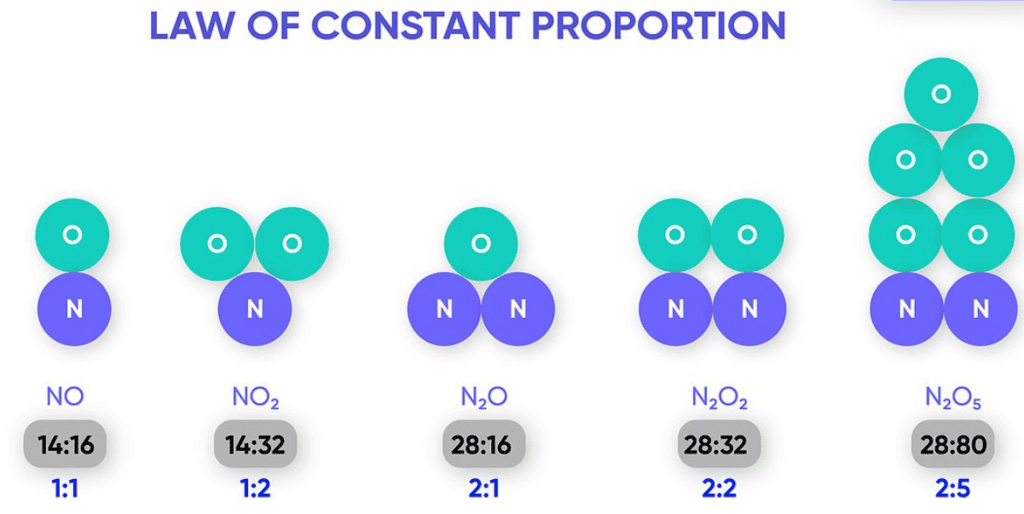 Example of Law of Constant Proportion
Example of Law of Constant Proportion
For example, water always contains hydrogen and oxygen in the same proportion 1 : 8 by mass, whatever the source of water, from the river, well or rainwater.
John Dalton's Atomic Theory
John Dalton's Atomic Theory, emerging as a result of these laws, introduces the concept of atoms as the fundamental particles of matter.
 John Dalton
John Dalton
Postulates of Atomic Theory
- All matter consists of atoms participating in chemical reactions.
- Atoms are indivisible and remain unchanged in reactions.
- Atoms of the same element are identical in mass and properties.
- Atoms of different elements have distinct masses and properties.
- Atoms combine in simple whole-number ratios to form compounds.
- The composition of atoms in a compound is constant.
Background on John Dalton
- Born in 1766, Dalton's atomic theory revolutionized the understanding of matter.
- His theory explained the Law of Conservation of Mass and the Law of Definite Proportions.
What is an Atom?
An atom is the defining structure of an element, which cannot be broken by any chemical means.
- Atoms are remarkably small, surpassing easy visualization.
- Stacking millions of atoms results in a layer as thin as a sheet of paper.
Atomic Radius: Measured in nanometers (1/109 meters = 1 nm).
What Are The Modern Day Symbols Of Atoms Of Different Elements?
Element symbols have evolved significantly over time, reflecting changes in scientific understanding and international standards. From Dalton's early use of symbols to represent atoms to the modern IUPAC-approved symbols, these notations now follow a standardized format that ensures clarity and consistency in the representation of elements.

- Historical Background: John Dalton was the first scientist to use symbols for elements, where each symbol represented a definite quantity, specifically one atom of the element. Berzilius suggested that element symbols be derived from one or two letters of the element's name.
- Origin of Element Names: Element Names are often derived from the place of discovery (e.g., copper from Cyprus). Some names reflected specific colors (e.g., gold from the English word for yellow).
- Modern Symbols: The International Union of Pure and Applied Chemistry (IUPAC) now approves element names and symbols. Most symbols are derived from the element's English name, using one or two letters. The first letter is always capitalized (uppercase), and the second letter, if present, is lowercase.
- First Letter + Another Letter: Symbols are sometimes formed from the first letter and another letter from the name (e.g., Chlorine: Cl, Zinc: Zn).
Here are some examples of symbols approved by IUPAC Symbols for Some Elements
Symbols for Some Elements - Latin, German, or Greek Roots: Some symbols come from the element's name in other languages:
Iron: Fe (from Latin "ferrum")
Sodium: Na (from Latin "natrium")
Potassium: K (from Latin "kalium")
Atomic Mass
Atomic mass is the total of the masses of the electrons, neutrons, and protons in an atom, or in a group of atoms, the average mass.
- The mass of an atomic particle is called the atomic mass.
- This is commonly expressed as per the international agreement in terms of a unified atomic mass unit (AMU).
- It can be best defined as 1/12 of the mass of a carbon-12 atom in its ground state.
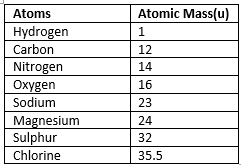 Atomic mass of some elements
Atomic mass of some elements
How Do Atoms Exist?
- Atoms of most elements cannot exist independently.
- Atoms form molecules and ions, and these aggregate to form visible matter.
What is a Molecule?
A molecule may be defined as the smallest particle of an element or a compound that is capable of independent existence and shows all the properties of that substance.
Molecules of Elements
- Monoatomic Molecules: Elements like Argon (Ar) and Helium (He) consist of only one atom of that element in their molecules.
- Diatomic Molecules: Nonmetals like Oxygen (O2), Hydrogen (H2), Nitrogen (N2), and Chlorine (Cl2) form molecules with two atoms of the same element, and this is known as diatomic molecules.
- Polyatomic Molecules: Some elements, like Phosphorus (P4) and Sulphur (S8), can form molecules consisting of more than two atoms. For example, phosphorus forms tetra-atomic molecules (P4), and sulphur forms polyatomic molecules.
Atomicity
The number of atoms constituting a molecule is known as its atomicity.
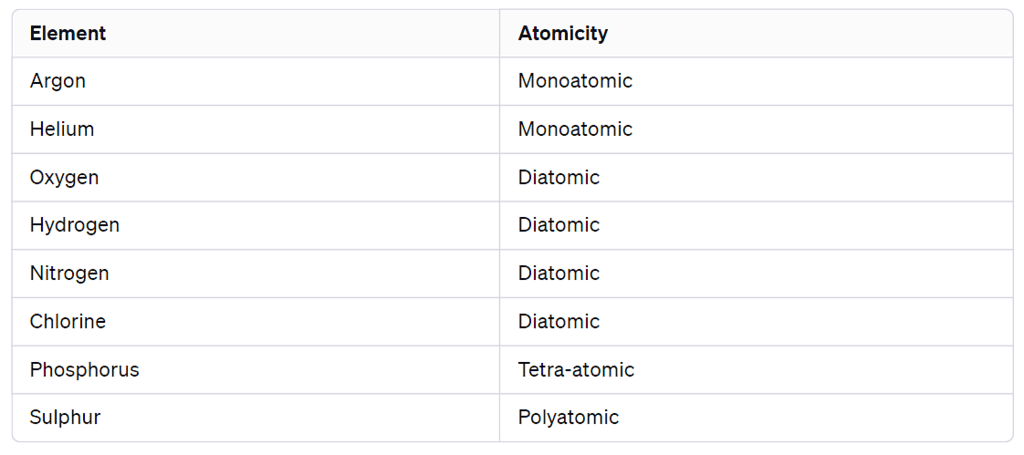 Atomicity of some non-metals
Atomicity of some non-metals
Molecules of Compounds
Atoms of different elements combine in definite proportions to form molecules of compounds.

What is an Ion?
- Compounds composed of metals and non-metals contain charged species called ions.
- Ions can be single-charged atoms or groups of atoms with a net charge.
- An ion can be positively charged (cation) or negatively charged (anion).
- Polyatomic ions are groups of atoms with a net charge.
Writing Chemical Formulae
- The chemical formula of a compound serves as a symbolic representation of its composition.
- To write chemical formulas, it is essential to understand the symbols and combining capacities (valencies) of the elements involved.
- The combining power or capacity of an element is termed its valency.
- This property determines how atoms of an element will combine with atoms of another element to form a chemical compound.
- Valency can be likened to the arms or hands of an atom.

Rules for Formula Writing
- The valencies or charges on ions must balance.
- In compounds with both a metal and a non-metal, the metal's name or symbol is written first.
- Examples: Calcium oxide (CaO), sodium chloride (NaCl), iron sulfide (FeS), and copper oxide (CuO).
- For compounds formed with polyatomic ions, the number of ions present is indicated by enclosing the ion's formula in brackets and writing the number of ions outside the bracket.
- Examples: Mg(OH)₂, NaOH.
Formulae of Simple Compound
To write the chemical formulae of binary compounds (made up of two different elements), one can use the valencies of the ions involved. The process involves a "crossover" of the valencies of the combining atoms. Example
- The formula of Carbon Tetrachloride (CCl₄): Carbon is a non-metal with a valency of 4, and chlorine is a non-metal with a valency of 1. The formula involves crossover: CCl₄.

- The formula of Magnesium Chloride (MgCl₂): Magnesium is a metal with a valency of 2, and chlorine is a non-metal with a valency of 1. The formula involves crossover: MgCl₂.

Molecular Mass
The molecular mass of a substance is the sum of the atomic masses of all the atoms in a molecule of that substance. It is expressed in atomic mass units (u).
Example 1:
(a) Calculate the relative molecular mass of water (H₂O).
(b) Calculate the molecular mass of HNO₃.
Solution:
(a) Atomic mass of hydrogen = 1u, oxygen = 16u. So, the molecular mass of water (2H + 1O) = 2 × 1 + 1 × 16 = 18u.
(b) Molecular mass of HNO₃ (H + N + 3O) = 1 + 14 + 3 × 16 = 63u.
Formula Unit Mass
Formula unit mass, calculated similarly to molecular mass, is applicable to substances with ions as constituent particles. It represents the sum of the atomic masses of all atoms in a formula unit of a compound.
Example 2: Calculate the formula unit mass of CaCl₂.
Solution: Atomic mass of Ca + (2 × atomic mass of Cl) = 40 + 2 × 35.5 = 40 + 71 = 111u.
|
84 videos|478 docs|60 tests
|
FAQs on Atoms and Molecules Class 9 Notes Science Chapter 3
| 1. What are the different laws of chemical combination? |  |
| 2. What is the difference between an atom and a molecule? |  |
| 3. How do you write chemical formulae for compounds? |  |
| 4. What is a molecular mass and how is it calculated? |  |
| 5. What are molecules of compounds and how do they differ from molecules of elements? |  |





















