NEET Previous Year Questions (2014-2025): Redox Reactions | Chemistry Class 11 PDF Download
2025
Q1: Consider the following compounds: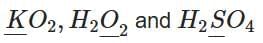
The oxidation states of the underlined elements in them are, respectively: (NEET 2025)
(a) +1, -2 and +4
(b) +4 , -4 and +6
(c) +1, -1 and +6
(d) +2, -2 and +6
Ans: (c)
In KO₂ (potassium superoxide):
- Potassium (K) has an oxidation state of +1.
- Oxygen in superoxide has an oxidation state of -½.
In H₂O₂ (hydrogen peroxide):
- Hydrogen (H) has an oxidation state of +1.
- Oxygen in peroxide has an oxidation state of -1.
In H₂SO₄ (sulfuric acid):
- Hydrogen (H) has an oxidation state of +1.
- Oxygen has an oxidation state of -2.
- To balance the molecule:
- 2(+1) + x + 4(-2) = 0
- x = +6
Therefore, sulfur (S) has an oxidation state of +6.
Therefore, the oxidation states of the underlined elements in KO₂, H₂O₂, and H₂SO₄ are respectively +1, -1, and +6.
2024
Q1: Which reaction is NOT a redox reaction? (NEET 2024)(a) Zn + CuSO4 → ZnSO4 + Cu
(b) 2KClO3 + I2 → 2KIO3 + Cl2
(c) H2 + Cl2 → 2HCl
(d) BaCl2 + Na2SO4 → BaSO4 + 2NaCl
Ans: (d)
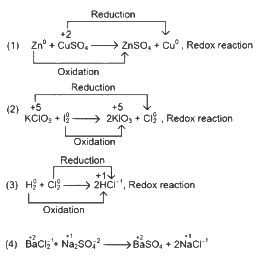
This is not a redox reaction as there is no change in oxidation state.
Q2: The products A and B obtained in the following reactions, respectively, are (NEET 2024)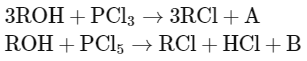
(a) 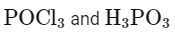
(b) 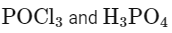
(c) 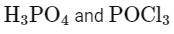
(d) 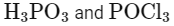
Ans: (d)
Let's analyze the given reactions and determine the products A and B.
Reaction with PCl3:
When an alcohol (ROH) reacts with phosphorus trichloride (PCl3), the hydroxyl group (OH) of the alcohol is replaced by a chloro group (Cl), resulting in the formation of an alkyl chloride (RCl). Additionally, the reaction produces phosphorous acid as a byproduct. This can be represented as:
3ROH + PCl3 → 3RCl + H3PO3
Here, A is H3PO3 (phosphorous acid).
Reaction with PCl5:
When an alcohol reacts with phosphorus pentachloride (PCl5), it also replaces the hydroxyl group with a chloro group. The reaction differs
slightly in the byproducts; phosphorus oxychloride (POCl3) and hydrogen chloride (HCl) are produced:
ROH + PCl5 → RCl + HCl + POCl3
Here, B is POCl3 (phosphorus oxychloride).
Thus, in the reactions provided:
A = H3PO3
B = POCl3
Matching these findings against the provided options, we see that Option D correctly identifies A as H3PO3 and B as POCl3:
Option D: H3PO3 and POCl3
This is the correct answer.
Q3: Which of the following reactions is not a disproportionation reaction? (NEET 2024)
(a) 2 F₂(g) + 2 OH⁻(aq) → 2 F⁻(aq) + OF₂(g) + H₂O(l)
(b) Cl₂(g)+ 2 OH⁻(aq) → ClO⁻(aq) + Cl⁻(aq) + H₂O(l)
(c) 2 NO₂(g) + 2 OH⁻(aq)→ NO₃⁻(aq) + NO₂⁻(aq) + H₂O(l)
(d) 2 H₂O₂(aq) → 2 H₂O(l) + O₂(g)
Ans: (a)
A disproportionation reaction is a type of redox reaction where a single substance undergoes both oxidation and reduction, forming two different products.
Let's analyze each option:
(a) 2 F₂(g) + 2 OH⁻(aq) → 2 F⁻(aq) + OF₂(g) + H₂O(l)
- This reaction involves fluorine (F₂), which is reduced to F⁻ and oxidized to OF₂. Therefore, fluorine undergoes both oxidation and reduction.
- This is a disproportionation reaction.
(b) Cl₂(g) + 2 OH⁻(aq) → ClO⁻(aq) + Cl⁻(aq) + H₂O(l)
- In this reaction, chlorine (Cl₂) is reduced to Cl⁻ and oxidized to ClO⁻.
- This is a disproportionation reaction.
(c) 2 NO₂(g) + 2 OH⁻(aq)→ NO₃⁻(aq) + NO₂⁻(aq) + H₂O(l)
- In this reaction, NO₂ undergoes both oxidation and reduction to form NO₃⁻ and NO₂⁻.
- This is a disproportionation reaction.
(d) 2 H₂O₂(aq)→ 2 H₂O(l) + O₂(g)
In this reaction, hydrogen peroxide (H₂O₂) decomposes into water (H₂O) and oxygen (O₂). Here, hydrogen peroxide undergoes reduction to form H₂O and oxidation to form O₂, but since it does not involve two different products from a single reactant, it is not a disproportionation reaction. It's a simple decomposition reaction.
Conclusion:The reaction in (a) is the disproportionation reaction, so the correct answer is (d) 2 H₂O₂(aq) → 2 H₂O(l) + O₂(g).
Q4: Given the following reaction involving manganese (Mn): (NEET 2024)
3 MnO₄²⁻ + 4 H⁺ → 2 MnO₄⁻ + MnO₂ + 2 H₂O
MnO₄²⁻ + 4 H⁺ → 2 MnO₄⁻ + MnO₂ + 2 H₂O
Which oxidation states of manganese are not observed in the above reaction?
A. +6
B. +2
C. +4
D. +7
E. +3
Mark the most appropriate answer from the options given below:
(a) D and E only
(b) B and D only
(c) A and B only
(d) B and E only
Ans: (d)
3 MnO₄²⁻ + 4 H⁺ → 2 MnO₄⁻ + MnO₂ + 2 H₂O
Step 1: Assign oxidation states in the reactants:
MnO₄²⁻: Manganese in MnO₄²⁻ is in the +6 oxidation state.
- Oxygen is typically -2, and since the overall charge on the ion is -2, the charge on Mn must be +6 to balance the charges.
- H⁺: This is a proton, so its oxidation state is +1.
Step 2: Assign oxidation states in the products:
MnO₄⁻: Manganese in MnO₄⁻ is in the +7 oxidation state.
Oxygen is -2, and since the overall charge on the ion is -1, the charge on Mn must be +7 to balance the charges.
MnO₂: Manganese in MnO₂ is in the +4 oxidation state.
Oxygen is -2, and for the compound to be neutral, Mn must have a charge of +4.
H₂O: Water, which does not affect the oxidation state of manganese.
Step 3: Identify which oxidation states are missing:
- +6 is observed in MnO₄²⁻.
- +7 is observed in MnO₄⁻.
- +4 is observed in MnO₂.
- +2 and +3 are not present in the reaction.
Conclusion:The +2 and +3 oxidation states of manganese are not observed in the given reaction.
The correct answer is (d) B and E only.
2023
Q1: On balancing the given redox reaction, (NEET 2023)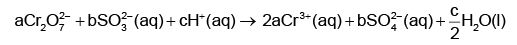
the coefficients a, b and c are found to be, respectively-
(a) 1, 3, 8
(b) 3, 8, 1
(c) 1, 8, 3
(d) 8, 1, 3
Ans: (a)
Using Ion electron method: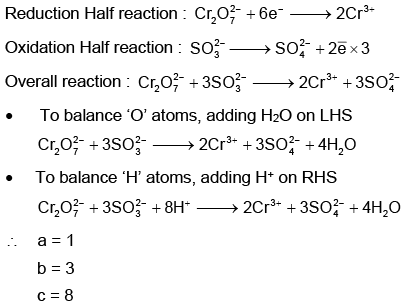
 Balancing Redox Reactions by Oxidation Number Method
Balancing Redox Reactions by Oxidation Number Method
2022
Q1: Which of the following reactions is a decomposition redox reaction? (NEET 2022 Phase 2)
(a) P4(s) + 3OH
(b) 2Pb(NO3)2(s)
(c) N2(g) + O2(g)
(d) Cl2(g) + 2OH
Ans: (b)
Decomposition redox reaction leads to breakdown of a compound into two or more compounds at least one of which must be in the elemental state with change in oxidation number.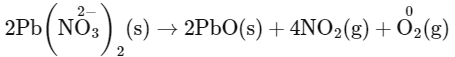
2020
Q1: What is the change in oxidation number of carbon in the following reaction?
CH4(g) + 4Cl2(g)
(a) 0 to + 4
(b) −4 to + 4
(c) 0 to − 4
(d) + 4 to + 4
Ans: (b)
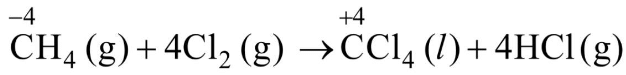
Change in oxidation state of carbon is from − 4 to + 4
2019
Q1: Which of the following reactions are disproportionation reaction? (NEET 2019)
(a) 2Cu+ → Cu2+ + Cu
(b) 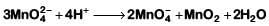
(c) 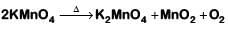
(d) 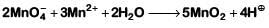
Select the correct option from the following
(a) (a) and (b) only
(b) (a), (b) and (c)
(c) (a), (c) and (d)
(d) (a) and (d) only
Ans: (a)
In a disproportionation reaction, same substance undergoes oxidation (increase in oxidation number) and reduction (decrease in oxidation number forming two different products.
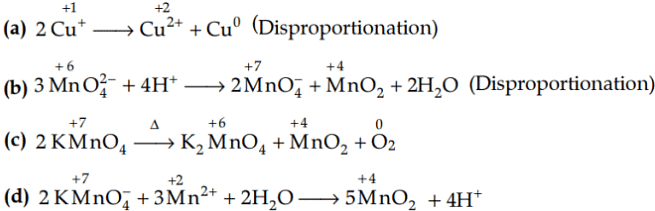
2018
Q1: The correct order of N-compounds in its decreasing order of oxidation states is (NEET 2018)
(a) HNO3, NO, N2, NH4Cl
(b) HNO3, NO, NH4Cl, N2
(c) HNO3, NH4Cl, NO, N2
(d) NH4Cl, N2, NO, HNO3
Ans: (a)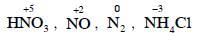
Q2: For the redox reaction
MnO4– + C2O42–+ H+ →Mn2+ + CO2 + H2O
the correct coefficients of the reactants for the balanced equation are (NEET 2018)
MnO4– C2O4 2– H+
(1) 16 5 2
(2) 2 5 16
(3) 2 16 5
(4) 5 16 2
(a) 1
(b) 2
(c) 3
(d) 4
Ans: (b)
The correct balanced equation is
2MnO4– + 5C2O4 2– + 16H+
2016
Q1: Hot concentrated sulphate acid is a moderately strong oxidizing agent. Which of the following reactions does not show oxidizing behaviour?
(a) Cu + 2H2SO4
(b) S + 2H2SO4
(c) C + 2H2SO4
(d) CaF2 + H2SO4
Ans: (d)
CaF2 + H2SO4
Here, the oxidation state of every atom remains the same so, it is not a redox reaction.
2014
Q1: The pair of compounds that can exist together is : (NEET 2014)
(a) HgCl2 ,SnCl2
(b) FeCl2 ,KI
(c) FeCl3,SnCl2
(d) FeCl2 ,SnCl2
Ans: (a)
The compounds with lower oxidation number and which cannot reduce by one another can exist together. Thus, FeCl2 and SnCl2 can exist together as Fe2+ can not be reduced by Sn2+.
Q2: In acidic medium H2O2 changes Cr2O7 to CrO5 which has two −O−O− bonds. Oxidation state of Cr in CrO5 is : (2014)
(a) +6
(b) -10
(c) +5
(d) +3
Ans: (a)
CrO5 has butterfly structure having two peroxo bonds.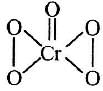
Peroxo oxygen has -1 oxidation state.
Let oxidation state of Cr be 'x'
CrO5 : x + 4(-1) + 1 (-2) = 0 ⇒ x = + 6
|
114 videos|263 docs|74 tests
|
FAQs on NEET Previous Year Questions (2014-2025): Redox Reactions - Chemistry Class 11
| 1. What is a redox reaction? |  |
| 2. How do you determine if a reaction is a redox reaction? |  |
| 3. What is the role of oxidizing and reducing agents in a redox reaction? |  |
| 4. Can you give an example of a redox reaction? |  |
| 5. How are redox reactions important in everyday life? |  |

















