NEET Previous Year Questions (2014-2025): Hydrogen (Old NCERT) | Chemistry Class 11 PDF Download
Q.1. Which of the following statements are NOT correct?
A. Hydrogen is used to reduce heavy metal oxides to metals.
B. Heavy water is used to study reaction mechanism.
C. Hydrogen is used to make saturated fats from oils.
D. The H–H bond dissociation enthalpy is lowest as compared to a single bond between two atoms of any elements.
E. Hydrogen reduces oxides of metals that are more active than iron.
Choose the most appropriate answer from the options given below:
(a) B, C, D, E only
(b) B, D only
(c) D, E only
(d) A, B, C only
Ans: c
Statement A, B, C are correct
(D) H – H bond dissociation energy is maximum as compared to single bond between two atom of any element.
(E) Hydrogen reduces oxides of metal that are less active than iron.
Q.2. Match List-I with List-II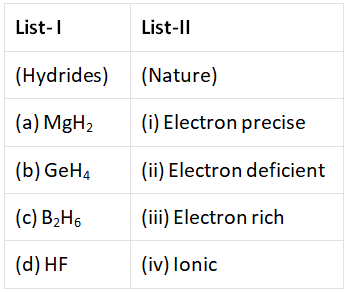
Choose the correct answer from the options given below: (2022)
(a) (a) – (i), (b) – (ii), (c) – (iv), (d) – (iii)
(b) (a) – (ii), (b) – (iii), (c) – (iv), (d) – (i)
(c) (a) – (iv), (b) – (i), (c) – (ii), (d) – (iii)
(d) (a) – (iii), (b) – (i), (c) – (ii), (d) – (iv)
Ans: c
MgH2 → Ionic
GeH4 → electron precise
B2H6 → electron deficient
HF → electron rich
Q.3. Tritium, a radioactive isotope of hydrogen, emits which of the following particles? (2021)
(a) Gamma (γ)
(b) Neutron (n)
(c) Beta (β)
(d) Alpha (α)
Ans: c
Tritium (13H or T) This isotope of hydrogen is radioactive and emits low energy β-particle.
It has one proton and two neutrons in the nucleus. The concentration of tritium is very low.
It is just about one atom per 1018 atoms of protium.
Q.4. Match the following and identify the correct option. (2020)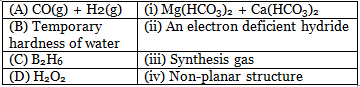
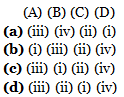
Ans: c
⇒ CO(g) + H2(g) = Water gas or synthesis gas
⇒ Temporary hardness of water is due to bicarbonates of Ca2+ & Mg2+
⇒ B2H6 is a electron deficient compound due to presence of banana bond.
⇒ H2O2 open book like structure which is non-planar
Q.5. The method used to remove temporary hardness of water is : (2019)
(a) Calgon's method
(b) Clark's method
(c) Ion-exchange method
(d) Synthetic resins method
Ans: b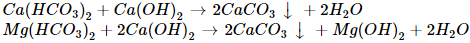
Clark's method is used to remove temporary hardness of water, in which bicarbonates of calcium and magnesium are reacted with slaked lime Ca(OH)2.
Q.6. Which of the following statements about hydrogen is incorrect ? (2016)
(a) Dihydrogen does not act as a reducing agent.
(b) Hydrogen has three isotopes of which tritium is the most common.
(c) Hydrogen never acts as cation in ionic salts.
(d) Hydronium ion, H3O+ exists freely in solution.
Ans: a,b
Dihydrogen act as reducing agent for eg: 3H2 + N2 → 2NH3
Hydrogen has three isotopes of which protium (1H1) is the most common.
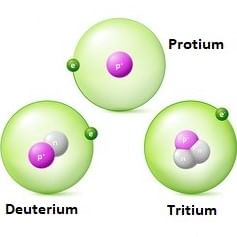 Isotopes of HydrogenQ.7. (A) H2O2 +O3 → H2O+ 2O2
Isotopes of HydrogenQ.7. (A) H2O2 +O3 → H2O+ 2O2
(B) H2O2 + Ag2O → 2Ag + H2O + O2
Role of hydrogen peroxide in the above reactions is respectively : (2014)
(a) reducing in (A) and (B)
(b) oxidizing in (A) and (B)
(c) oxidizing in (A) and reducing in (B)
(d) reducing in (A) and oxidizing in (B)
Ans: a
H2O2 acts as reducing agent in both the reactions
H2O2 +O3 → H2O+ 2O2
H2O2 + Ag2O → 2Ag + H2O + O2
|
114 videos|263 docs|74 tests
|





















