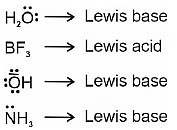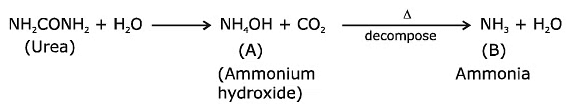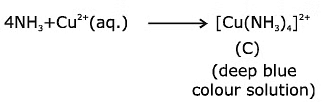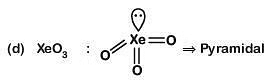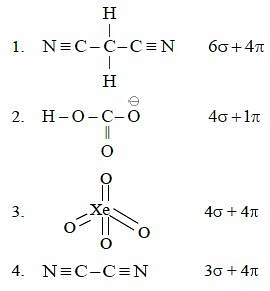NEET Previous Year Questions (2014-2025): The p-Block Elements (Group 13 & 14) | Chemistry Class 11 PDF Download
2024
Q1: Identify the Incorrect statement: (NEET 2024)
(a) Carbon has the tendency to form chains and rings with itself.
(b) Silicon and Germanium are useful in the semiconductor industry.
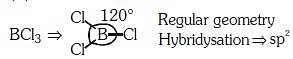
(c) Boron trifluoride is a strong Lewis acid.
(d) Common oxidation states of group 14 elements are +1 and +3.
Ans: (d)
Statement (a): Carbon is well-known for its ability to form chains and rings, making it unique among elements. It forms stable covalent bonds with itself, leading to a variety of organic compounds. Hence, Statement (a) is correct.
Statement (b): Silicon and Germanium are indeed useful in the semiconductor industry. They are commonly used in electronics due to their semiconductor properties. Hence, Statement (b) is correct.
Statement (c): Boron trifluoride (BF₃) is indeed a strong Lewis acid. It can accept electron pairs due to its incomplete octet. Hence, Statement (c) is correct.
Statement (d): The common oxidation states of group 14 elements (C, Si, Ge, Sn, Pb) are +4 and -4, not +1 and +3. +1 and +3 are more common oxidation states for group 13 elements (such as Boron, Aluminum, etc.).Hence, Statement (d) is incorrect.
Q2: Identify the incorrect statement: (NEET 2024)
(a) The relative stability of group 13 elements in +1 oxidation state varies as: Al < Ga < In < Tl.
(b) Boron trioxide is acidic; aluminium and gallium trioxides are amphoteric, while indium and thallium trioxides are basic.
(c) The hybridization of Al in [Al(H₂O)₆]³⁺ is d²sp³.
(d) Two isotopes of Boron, namely ¹⁰B (19%) and ¹¹B (81%), are known.
Ans: (c)
(a) The relative stability of group 13 elements in +1 oxidation state varies as: Al < Ga < In < Tl.
This statement is incorrect. The correct order for the stability of the +1 oxidation state in group 13 elements is Al > Ga > In > Tl. The stability of the +1 oxidation state decreases down the group, and thallium (Tl) shows the least stability due to the inert pair effect, where the s-electrons are more tightly bound and not easily ionized.(b) Boron trioxide is acidic; aluminium and gallium trioxides are amphoteric, while indium and thallium trioxides are basic.
This statement is incorrect. Boron trioxide (B₂O₃) is acidic, aluminium oxide (Al₂O₃) and gallium oxide (Ga₂O₃) are amphoteric, but indium oxide (In₂O₃) and thallium oxide (Tl₂O₃) are amphoteric as well, not basic. They can react with both acids and bases.(c) The hybridization of Al in [Al(H₂O)₆]³⁺ is d²sp³.
This statement is incorrect. The correct hybridization of Al in [Al(H₂O)₆]³⁺ is sp³d², not d²sp³. The d-orbitals are involved in the hybridization, but the correct order is sp³d².(d) Two isotopes of Boron, namely ¹⁰B (19%) and ¹¹B (81%), are known.
This statement is correct. Boron has two naturally occurring isotopes: ¹⁰B (19%) and ¹¹B (81%).
Conclusion: The incorrect statement is (c). The hybridization of Al in [Al(H₂O)₆]³⁺ is sp³d², not d²sp³.
Therefore, the correct answer is (c).
2023
Q.1. Taking stability as the factor, which one of the following represents correct relationship? (NEET 2023)
(a) TℓCI3 > TlCI
(b) InI3 > InI
(c) AlCl > AlCl3
(d) TℓI > TℓI3
Correct Answer is Option (D)
As we move down the group, due to poor shielding effect of intervening d and f orbitals, the increased effective nuclear charge holds ns electrons tightly and therefore restricting their participation in bonding.
So, the relative stability of +1 O.S increases for heavier elements.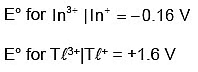
Hence, TℓI is more stable than TℓI3
Q.2. Amongst the given options which of the following molecules/ ion acts as a Lewis acid? (NEET 2023)
(a) NH3
(b) H2O
(c) BF3
(d) OH–
Correct Answer is Option (C)
Lewis acids are the one which accepts lone pair of electron due to presence of vacant orbital in outermost shell.
Q.3. Match List-I with List-II. (NEET 2023)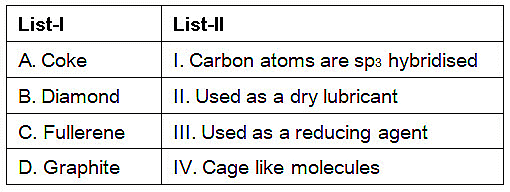
Choose the correct answer from the options given below:
(a) A-II, B-IV, C-I, D-III
(b) A-IV, B-I, C-II, D-III
(c) A-III, B-I, C-IV, D-II
(d) A-III, B-IV, C-I, D-II
Correct Answer is Option (C)
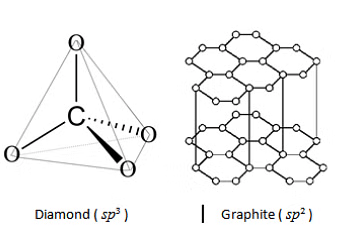
- Coke is largely used as a reducing agent in metallurgy.
- In diamond, each carbon atom undergoes sp3 hybridisation and linked to four other carbon atoms by using hybridised orbitals in tetrahedral fashion.
- Buckminsterfullerene contains six membered and five membered rings and hence is a cage like molecule.
- Graphite is very soft and slippery. Hence, it is used as a dry lubricant in machines running at high temperature.
Buckminsterfullerene
Q.4. Match List-I with List-II: (NEET 2023)
Choose the correct answer from the options given below.
(a) A–I, B–III, C–II, D–IV
(b) A–III, B–IV, C–I, D–II
(c) A–I, B–III, C–IV, D–II
(d) A–III, B–IV, C–II, D–I
Correct Answer is Option (B)
Q5: Select the element (M) whose trihalides cannot be hydrolyzed to form [M(H₂O)₆]³⁺: (NEET 2023)
(a) Ga
(b) In
(c) Al
(d) B
Ans: (d)
Trihalides of elements from group 13 (like Al, Ga, In) are capable of hydrolyzing in water to form a hydrated metal complex like [M(H₂O)₆]³⁺, where M is the metal in the +3 oxidation state.
Boron (B), however, does not hydrolyze in the same manner. Boron trifluoride (BF₃) is a strong Lewis acid, but it does not hydrolyze to form a similar hydrated ion as [M(H₂O)₆]³⁺ because boron does not readily form a stable +3 oxidation state in aqueous solution.
On the other hand, Al³⁺, Ga³⁺, and In³⁺ all form stable [M(H₂O)₆]³⁺complexes when their trihalides are hydrolyzed.Thus, the element whose trihalides cannot be hydrolyzed to form [M(H₂O)₆]³⁺ is Boron (B).
Q6: The EΘ values for (NEET 2023)
Al⁺/Al = +0.55 V and Tl⁺/Tl = -0.34 V
Al³⁺/Al = -1.66 V and Tl³⁺/Tl = +1.26 V
The incorrect statement among the following is:
(a) Al is more electropositive than Tl.
(b) Tl³⁺ is a good reducing agent than Tl¹⁺.
(c) Al³⁺ is unstable in solution.
(d) Tl can be easily oxidized to Tl³⁺ as compared to Tl¹⁺.
Ans: (b)
EΘ values for the reactions:
- Al⁺/Al = +0.55 V
- Tl⁺/Tl = -0.34 V
- Al³⁺/Al = -1.66 V
- Tl³⁺/Tl = +1.26 V
Statement analysis:
(a) Al is more electropositive than Tl.
- Electropositivity is the tendency of an element to lose electrons. An element with a more negative E° value for the reduction half-reaction is more electropositive because it prefers to lose electrons easily. Since Al⁺/Al has a higher (more positive) E° value than Tl⁺/Tl, Al is more electropositive than Tl.
This statement is correct.(b) Tl³⁺ is a good reducing agent than Tl¹⁺.
- A reducing agent is a species that donates electrons, and a good reducing agent has a more negative E° value because it is easier for the species to lose electrons. Here, Tl³⁺/Tl has a positive E° value (+1.26 V), meaning Tl³⁺ is easily reduced to Tl. Tl⁺/Tl, with a negative E° value (-0.34 V), suggests that Tl⁺ is more easily reduced (and thus Tl⁺ is a better oxidizing agent).Tl³⁺ is not a better reducing agent than Tl¹⁺ because Tl³⁺ is more easily reduced (acts as an oxidizing agent).This statement is incorrect.
(c) Al³⁺ is unstable in solution.
- The E° for Al³⁺/Al is -1.66 V, which is quite negative. This indicates that Al³⁺ is highly unstable in solution and is readily reduced to Al metal.
This statement is correct.(d) Tl can be easily oxidized to Tl³⁺ as compared to Tl¹⁺.
- The E° for Tl³⁺/Tl is positive (+1.26 V), meaning it is easier for Tl to be oxidized to Tl³⁺ than to Tl¹⁺ (because oxidation is the reverse of reduction, and a positive E° favors oxidation).
This statement is correct.Conclusion: The incorrect statement is (b): Tl³⁺ is a good reducing agent than Tl¹⁺.
Thus, the correct answer is (b).
2022
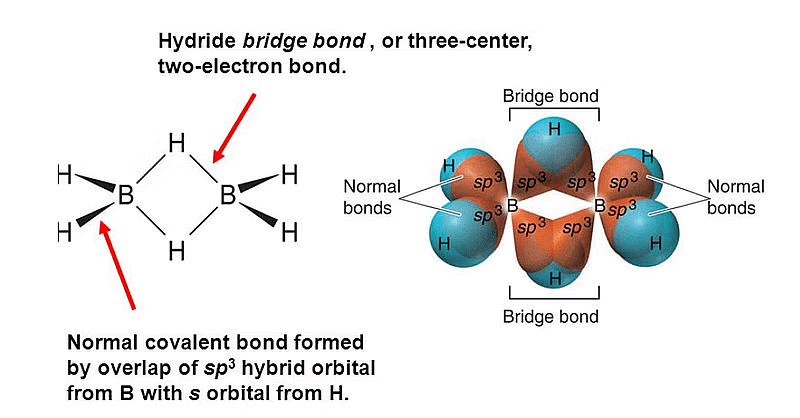
Q.5. Which of the following statement is not correct about diborane? (2022)
(a) The four terminal Hydrogen atoms and the two Boron atoms lie in one plane.
(b) Both the Boron atoms are sp2 hybridised.
(c) There are two 3-centre-2-eIectron bonds.
(d) The four terminal B-H bonds are two centre two electron bonds.
Correct Answer is Option (B)
In diborane both the boran atoms are sp3 hybridized.
Q.6. Given below are two statements: one is labelled as Assertion (A) and the other is labelled as Reason (R).
Assertion (A): ICl is more reactive than I2.
Reason (R): ICl bond is weaker than I-I bond.
In the light of the above statements, choose the most appropriate answer from the options given below: (2022)
(a) (A) is correct but (R) is not correct.
(b) (A) is not correct but (R) is correct.
(c) Both (A) and (R) are correct and (R) is the correct explanation of (A).
(d) Both (A) and (R) are correct but (R) is not the correct explanation of (A).
Correct Answer is Option (C)
I2 forms covalent bond which is more stronger than inter-halogen compound and weak bonds are obviously more reactive than stronger bond
∴ ICl is more reactive than I2 as I2 forms stronger bond than ICl.
Q.7. Choose the correct statement: (2022)
(a) Diamond is sp3 hybridised and graphite is sp2 hybridized.
(b) Both diamond and graphite are used as dry lubricants.
(c) Diamond and graphite have two dimensional network.
(d) Diamond is covalent and graphite is ionic.
Correct Answer is Option (A)
In diamond carbon atom is in sp3 hybridisation In graphite carbon atom is in sp2 hybridisation.
2021
Q.8. Statement I: Acid strength increases in the order given as HF << HCl << HBr << HI.
Statement II: As the size of the elements F, Cl, Br, I increase down the group, the bond strength of HF, HCI, HBr, and HI decreases and so the acid strength increases.
In the light of the above statements, choose the correct answer from the options given below. (2021)
(a) Statement I is correct but Statement II is false.
(b) Statement I is incorrect but Statement II is true.
(c) Both Statement I and Statement II are true.
(d) Both Statement I and Statement II are false.
Ans: (C) Both statements I and II are correct.
Solution: Acid strength depends on two factors which is Electronegativity and Bond Strength
- Electronegativity: As electronegativity increases acid strength will also increase.
- Bond strength: Longer the bond, the weaker it will be, hence if bond strength is weaker acid will be stronger.
In the given question: Acid strength increases in the order given as HF << HCl << HBr << HI. Because as we move from Fluorine to Iodine, the size of halogen increases. The larger the size of the halogen longer the bond will be formed hence bond strength will decrease means it requires less energy for bond dissociation and can be easily broken. So, HI is the stronger acid of all.
Both statements I and II are correct.
Q.9. The incorrect statement about noble gases is - (2021)
(a) Noble gases have weak dispersion forces.
(b) Noble gases have large positive values of electron gain enthalpy.
(c) Noble gases are sparingly soluble in water.
(d) Noble gases have very high melting and boiling points.
Correct Answer is Option (D)
Solution: Noble gases have weak dispersion forces so their melting and boiling point are very low.
Q.10. In which one of the following arrangements the given sequence is not strictly according to the properties indicated against it? (2021)
(a) NH3 < PH3 < AsH3 < SbH3 : Increasing acidic character
(b) CO2 < SiO2 < SnO2 < PbO2 : Increasing oxidizing power
(c) HF < HCl < HBr < HI : Increasing acidic strength
(d) H2O < H2S < H2Se < H2Te : Increasing pKa values
Correct Answer is Option (D)
Solution:
The following sequence is not strictly according to the property indicated against it.
H2O<H2S<H2Se<H2Te; increasing pKavalues.The correct sequence is:
H2O>H2S>H2Se>H2Te; decreasing pKa values.In Hydrides, as we move top to bottom, acidic nature increases so with the increase in the acidity from top to bottom, the Ka value increases and pKa value decreases.
2020
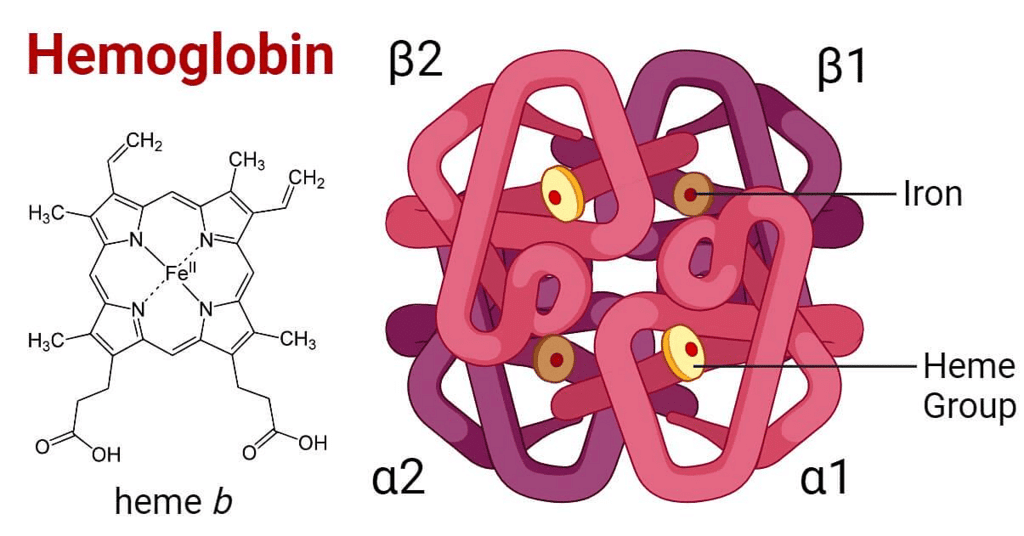
Q.11. Which of the following is not correct about carbon monoxide? (2020)
(a) The carboxyhaemoglobin (haemoglobin bound to CO) is less stable than oxyhaemoglobin.
(b) It is produced due to incomplete combustion.
(d) It forms carboxyhaemoglobin.
(d) It reduces oxygen carrying ability of blood.
Correct Answer is Option (A)
CO when combine with haemoglobin it forms carboxyhaemoglobin which more stable than oxyhaemoglobin thats why it reduces oxygen carring ability of blood.
Q.12. Identify the correct statements from the following: (2020)
(a) CO2(g) is used as refrigerant for ice-cream and frozen food.
(b) The structure of C60 contains twelve six carbon rings and twenty five carbon rings.
(c) ZSM-5, a type of zeolite, is used to convert alcohols into gasoline.
(d) CO is colourless and odourless gas.
(a) (b) and (c) only
(b) (c) and (d) only
(c) (a), (b) and (c) only
(d) (a) and (c) only
Correct Answer is Option (B)
⇒ CO2 (s) is not used as refrigerant for ice-cream & frozen food.⇒ ZSM-5 zeolite used to convert alcohols into gasoline
⇒ CO is colourless & odourless gas
Q.13. Match the following : (2020)
Which of the following is correct option?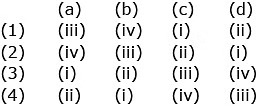
(a) (1)
(b) (2)
(c) (3)
(d) (4)
Correct Answer is Option (D)
Solution:
CO - Neutral
BaO - Basic
Al2O3 - Amphoteric
Cl2O7 - Acidic
Q.14. Urea reacts with water to form A which will decompose to form B. B when passed through Cu2+ (aq), deep blue colour solution C is formed. What is the formula of C from the following? (2020)
(d) Cu(OH)2
(b) CuCO3.Cu(OH)2
(c) CuSO4
(d) [Cu(NH3)4]2+
Correct Answer is Option (D)
Solution:
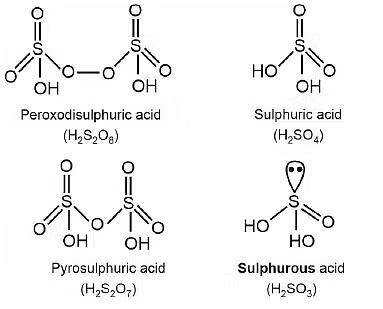
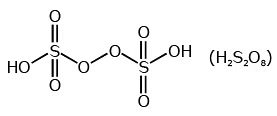
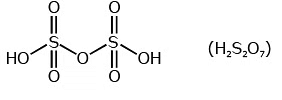
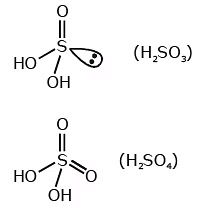
Q.15. Which of the following oxoacid of sulphur has –O–O– linkage? (2020)
(a) H2S2O8, peroxodisulphuric acid
(b) H2S2O7, pyrosulphuric acid
(c) H2SO3, sulphurous acid
(d) H2SO4, sulphuric acid
Correct Answer is Option (A)
Solution:
2019
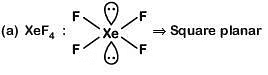
Q.16. Match the Xenon compounds in Column-I with its structure in Column-II and assign the correct code: (2019)
(a) (1)
(b) (2)
(c) (3)
(d) (4)
Correct Answer is Option (B)
Q.17. Which is the correct thermal stability order for H2E (E = O, S, Se, Te and Po)? (2019)
(a) H2S < H2O < H2Se < H2Te < H2Po
(b) H2O < H2S < H2Se < H2Te < H2Po
(c) H2Po < H2Te < H2Se < H2S < H2O
(d) H2Se < H2Te < H2Po < H2O < H2S
Correct Answer is Option (C)
On going down the group thermal stability order for H2E decreases because H–E bond energy decreases
∴ Order of stability would be:-
H2Po < H2Te < H2Se < H2S < H2O
Q.18. Among the following, the one that is not a green house gas is (2019)
(a) Nitrous oxide
(b) Methane
(c) Ozone
(d) Sulphur dioxide
Correct Answer is Option (D)
Fact: SO2 (g) is not a greenhouse gas.
Q.19. Which of the following species is not stable? (2019)
(a) [SiF6]2-
(b) [GeCl6]2-
(c) [Sn(OH)6]2-
(d) [SiCl6]2-
Correct Answer is Option (D)
Due to presence of d-orbital in Si, Ge and Sn they form species like SiF62-, [GeCl6]2-, [Sn(OH)6]2-
SiCl62- does not exist because six large chloride ions cannot be accommodated around Si4+ due to limitation of its size.
2018
Q.20. Which of the following statements is not true for halogens ? (2018)
(a) All form monobasic oxyacids.
(b) All are oxidizing agents.
(c) All but fluorine show positive oxidation states.
(d) Chlorine has the highest electron-gain enthalpy.
Correct Answer is Option (C)
Due to high electronegativity and small size, F forms only one oxoacid, HOF known as Fluoric (I) acid. Oxidation number of F is +1 in HOF.
Q.21. In the structure of ClF3, the number of lone pairs of electrons on central atom 'Cl' is (2018)
(a) one
(b) two
(c) three
(d) four
Correct Answer is Option (B)
2 lone pairs at equitorial position.
Q.22. Which one of the following elements is unable to form MF63- ion? (2018)
(a) Ga
(b) AI
(c) B
(d) In
Correct Answer is Option (C)
Boron belongs to 2nd period and it does not have vacant d-orbital.
Q.23. The correct order of atomic radii in group 13 elements is (2018)
(a) B < Al < In < Ga < Tl
(b) B < Al < Ga < In < Tl
(c) B < Ga < Al < Tl < In
(d) B < Ga < Al < In < Tl
Correct Answer is Option (D)
Atomic and ionic radii. Atomic and ionic radii of group 13 elements increase from top to bottom in the group. This is due to increase in the number of energy shells in each succeeding element. However, the atomic radius of gallium (Ga) is less than that of aluminium (Al).
It is due to the poor shielding of the valence electrons of Ga by the inner 3d-electrons. As a result, the effective nuclear charge of Ga is somewhat greater in magnitude than that of Al. Thus, the electrons in gallium experience the greater force of attraction by the nucleus than in aluminium. Hence the atomic size of Ga(135 pm) is slightly less than that of Al(143 pm).
2017
Q.24. Match the interhalogen compounds of column-I with the geometry in column II and assign the correct code. (2017)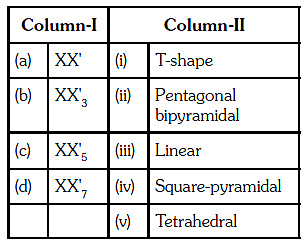
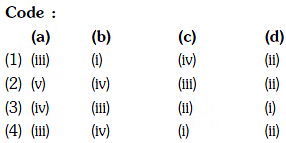
(a) (1)
(b) (2)
(c) (3)
(d) (4)
Correct Answer is Option (A)
(a)
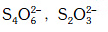
(b)
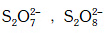
(c)
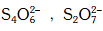
(d)
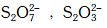
Correct Answer is Option (A)
(a) CIF3
(b) NCl3
(c) BCl3
(d) PH3
Correct Answer is Option (C)
(a) Sn2+ is reducing while Pb4+ is oxidising
(b) Sn2+ is oxidising while Pb4+ is reducing
(c) Sn2+ and Pb2+ are both oxidising and reducing
(d) Sn4+ is reducing while Pb4+ is oxidising
Correct Answer is Option (A)
Inability of ns2 electrons of the valence shell to participate in bonding on moving down the group in heavier p-block elements is called inert pair effect
As a result, Pb(II) is more stable than Pb(IV)
Sn(IV) is more stable than Sn(II)
∴ Pb(IV) is easily reduced to Pb(II)
∴ Pb(IV) is oxidising agent
Sn(II) is easily oxidised to Sn(IV)
∴ Sn(II) is reducing agent
2016
Q.28. When copper is heated with conc. HNO3 it produces : (2016)
(a) Cu(NO3)2 and N2O
(b) Cu(NO3)2 and NO2
(c) Cu(NO3)2 and NO
(d) Cu(NO3)2 , NO and NO2
Correct Answer is Option (B)
Nitric acid acts as an oxidising agent while reacting with copper.
i) when copper reacts with dilute nitric acid it forms,
3Cu + 4HNO3(dilute) → 3Cu(NO3)2 +2NO +4H2O
ii) When copper reacts with concentrated nitric acid it forms,
Cu + 4HNO3(conc.) → Cu(NO3)2 +NO2 +2H2O
Q.29. Which is the correct statement for the given acids? (2016)
(a) Phosphinic acid is a diprotic acid while phosphonic acid is a monoprotic acid.
(b) Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid.
(c) Both are diprotic acids.
(d) Both are triprotic acids.
Correct Answer is Option (B)
Phosphinic acid is a monoprotic acid while phosphonic acid is a diprotic acid.
Phosphinic acid-Phosphonic acid-
Due to the presence of one replaceable proton in phosphinic acid, it is monoprotic acid and due to the presence of two replaceable proton in phosphinic acid, it is diprotic acid.
Q.30. Among the following, the correct order of acidity is : (2016)
(a) HCIO4 < HCIO2 < HCIO < HCIO3
(b) HCIO3 < HCIO4 < HCIO2 < HCIO
(c) HCIO < HCIO2 < HCIO3 < HCIO4
(d) HCIO2 < HCIO < HCIO3 < HCIO4
Correct Answer is Option (C)
As oxidation number of central atom increases, acidic nature increases.
HClO < HClO2 < HClO3 < HClO4
Q.31. The product obtained as a result of a reaction of nitrogen with CaC2 is : (2016)
(a) Ca2CN
(b) Ca(CN)2
(c) CaCN
(d) CaCN3
Correct Answer is Option (B)
When calcium carbide reacts with nitrogen under high temperature, it forms calcium cyanamide which is also called nitrolim.
Q.32. Which one of the following orders is correct for the bond dissociation enthalpy of halogen molecules? (2016)
(a) F2 > Cl2 > Br2 > I2
(b) I2 > Br2 > CI2 > F2
(c) CI2 > Br2 > F2 > I2
(d) Br2 > I2 > F2 > CI2
Correct Answer is Option (C)
Bond dissociation energies of halogen family decrease down the group as the size of the atom increases. The bond dissociation energy of fluorine, is, however, lower than those of chlorine and bromine because of interelectronic repulsions present in the small atom of fluorine.
Hence bond energy decreases in the order Cl2 > Br2 > F2 > I2
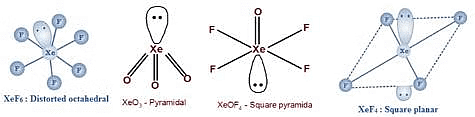
Q.33. Match the compound given in column I with the hybridization and shape given in column II and mark the correct option. (2016)
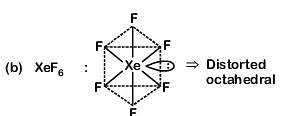
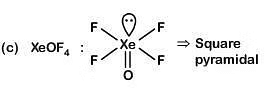
Column-I Column-II
(a) XeF6 (i) distorted octahedral
(b) XeO3 (ii) square planar
(c) XeOF4 (iii) pyramidal
(d) XeF4 (iv) square pyramidal
Code :
(a) (b) (c) (d)
(a) (iv) (i) (ii) (iii)
(b) (i) (iii) (iv) (ii)
(c) (i) (ii) (iv) (iii)
(d) (iv) (iii) (i) (ii)
Correct Answer is Option (B)
(i) (iii) (iv) (ii)
2015
Q.34. Which of the following species contain equal number of σ - and π - bonds ? (2015)
(a) CH2(CN)2
(b)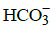 (c) XeO4
(c) XeO4
(d) (CN)2
Correct Answer is Option (C)
Q.35. Nitrogen dioxide and sulphur dioxide have some properties in common. Which property is shown by one of these compounds, but not by the other ? (2015)
(a) is used as a food-preservative
(b) forms 'acid-rain'
(c) is a reducing agent
(d) is soluble in water
Correct Answer is Option (A)
SO2 is used in the manufacture of sodium bisulphate (NaHSO3) which is used as preservatives for jams, jellies and squashes.
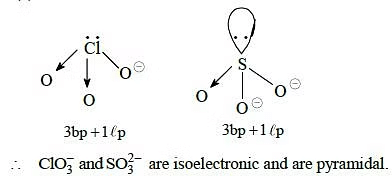
Q.36. Which of the following pairs of ions are isoelectronic and isostructural ? (2015)
(a)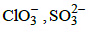 (b)
(b)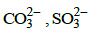 (c)
(c)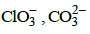 (d)
(d)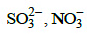
Correct Answer is Option (A)
Q.37. Maximum bond angle at nitrogen is present in which of the following ? (2015)
(a)
(b) NO2
(c)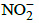
(d)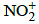
Correct Answer is Option (D)
In all of the four molecules NO2- and NO2 have one lone pair thus bond angle is less than 1200 in the case of NO2- and more than 1200 in the case of NO2 but in the NO3- there is no lone pair hence all are bonding pairs leading to an ideal bond angle of 1200.
In NO2+, the is no lone pair only bond pair exist thus leading a bond angle is 1800.
2014
(a) H2Te < H2S < H2Se
(b) H2Se < H2Te < H2S
(c) H2S < H2Se < H2Te
(d) H2Se < H2S < H2Te
Correct Answer is Option (C)
Acidic strength of hydrides increases as the size
of central atom increases which weakens the
M- H bond. Since, the size increases from S to Te
thus acidic strength follows the order.
H2S<H2Se<H2Te
Acidic nature ∝S to Te size increases, bond dissociation enthalpydecreases and acidic nature increases.
|
119 videos|346 docs|74 tests
|
FAQs on NEET Previous Year Questions (2014-2025): The p-Block Elements (Group 13 & 14) - Chemistry Class 11
| 1. What are the general electronic configurations of Group 13 and Group 14 elements in the periodic table? |  |
| 2. How does the atomic size of Group 13 elements change down the group? |  |
| 3. Why do Group 14 elements show a greater tendency to form covalent compounds compared to Group 13 elements? |  |
| 4. How do the melting and boiling points of Group 14 elements compare to those of Group 13 elements? |  |
| 5. Explain the trend in oxidation states exhibited by Group 13 and Group 14 elements in the periodic table. |  |