CARBOCATION
Properties of Carbocation
- It is positively charged species.
- It has sixtet of electrons i.e. diamagnetic.
- It is formed by heterolysis.
- It is generally formed due to polar solvent.
Structure: (sp2), Triangular planar
Reaction Intermediates: Reactive, shortlived, high energy, unstable species, those are formed in the course of organic reactions called reaction intermediates.
Reaction intermediates generally formed alter bond breaking and before bond formation.
A covalent bond can get cleaved either by
- Heterolytic cleavage, or
- Homolytic cleavage.
Homolytic fission of Covalent Bonds: The bond may break in such a way that each fragment takes away one of the electrons of the bond. This process called homolysis, produces fragments with unpaired electrons called radicals. Heterolytic fission of Covalent Bonds: The bond may break in such a way that one fragment takes away both electrons of the bond, leaving the other fragment with an empty orbital. This kind of cleavage called heterolysis, produces charged fragments or ions.
Heterolytic fission of Covalent Bonds: The bond may break in such a way that one fragment takes away both electrons of the bond, leaving the other fragment with an empty orbital. This kind of cleavage called heterolysis, produces charged fragments or ions. Heterolysis of a bond normally requires that the bond be polarised.
Heterolysis of a bond normally requires that the bond be polarised.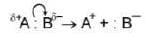 Polarisation of a bond usually result from different electronegatives of the atoms joined by the bond. The greater the difference in electronegativity, the greater the polarisation. In the given instance, atom B is more electronegative than A.
Polarisation of a bond usually result from different electronegatives of the atoms joined by the bond. The greater the difference in electronegativity, the greater the polarisation. In the given instance, atom B is more electronegative than A.
(a) Carbanion: A carbon intermediate which contain three bond pair and a negative charge on it, is called carbanion.
Hybridisation: Hybridisation of carbanion may be sp3, sp2 & sp.
| Hybridisation | Example |
| sp3 | 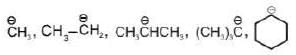 |
| sp2 | 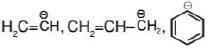 |
| sp | 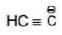 |
Stability of carbanion: Carbanions are stabilised by electron withdrawing effect as
(i) - I effect
(ii) - m effect
(iii) Delocalisation of charge
Carbanions are Lewis bases In their reactions they seek a proton or some other positive centre to which they can donate their electron pair and thereby neutralize their negative charge.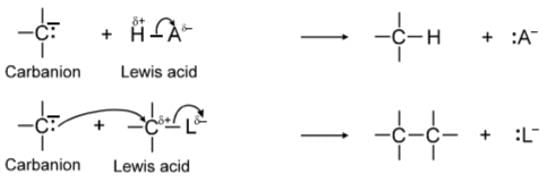
Example of stability order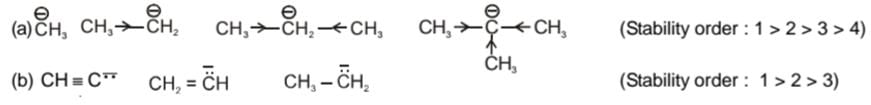
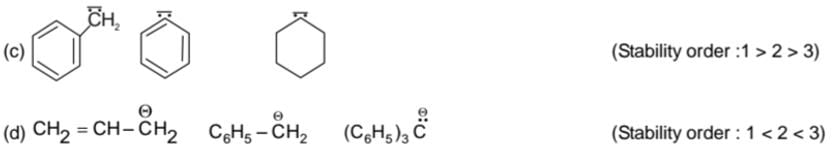
(b) Free Radicals:
Homolysis of covalent bond results into free radical intermediates possess the unpaired electrons. It is generated in presence of Sun light. Peroxides or High temperature
It is generated in presence of Sun light. Peroxides or High temperature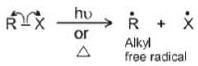 Free Radical: An uncharged intermediate which has three bond pair and an unpaired electron on carbon.
Free Radical: An uncharged intermediate which has three bond pair and an unpaired electron on carbon.
Note
- It is Neutral species with odd e-
- It is paramagnetic in nature due to odd e-
- No rearrangement is observed generally.
- Carbon atom having odd electron is in sp2 hybridised state
- Any reaction if it is carried out in the presence of sunlight, peroxide or high temperature it generally proceeds via free radical intermediate.
Stability of free radical: It is stabilised by resonance, hyperconjugation and + 1 groups.
Example:
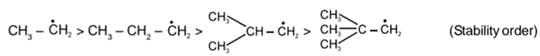
(due to resultant of inductive effect and hyperconjugation, both operates in same direction)
Example: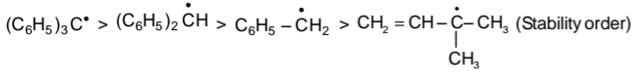
| Carbon free radical | Carbocation | Carbanion | |
 | 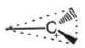 | 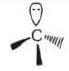 | |
| Shape | trigonal planar | trigonal planar | Pyramidal |
| Hybridisation | sp2 | sp2 | sp3 |
| No. of electrons in outermost shell | 7 | 6 | 8 |
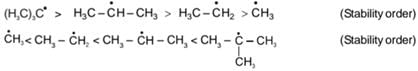
Example: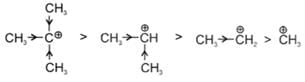 t-Butyl carbocation has +I effect of three Me-groups and also Hyperconjugation effect which makes it most stable.
t-Butyl carbocation has +I effect of three Me-groups and also Hyperconjugation effect which makes it most stable.
Example: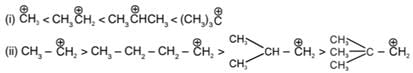
(due to resultant of inductive effect and hyperconjugabon)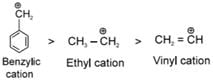
In Benzyl cation, Extensive Resonance ts seen which stabilises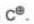
In Ethyl carbocation +I and Hyperconjugabon of Me - group stabiizes carbocation.
In vinyl carbocation stability decreases rapidly since carbon of (CH2) is sp2 hybridized which ts slightly more electronegative hence acts as -I group which increases (+) charge density.
(c) Carbocation: A carbon intermediate which contain three bond pair & a positive charge on it is called carbocation.
Hybridisation: Carbocation may be sp2 & sp hybridised
| Hybridisation | Example |
| sp2 | 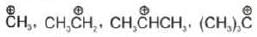 |
| sp | 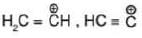 |
Carbocations are electron deficient. They have only six electrons in their valence shell, and because of this, carbocations act as Lewis acids. Most of the carbocations are short-lived and highly reactive, they occur as intermediates in some organic reactions. Carbocations react with Lewis bases or ions that can donate the electron pair, that they need to achieve a stable octet of electrons (i.e., the electronic configuration of a noble gas):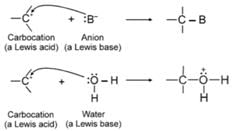
Because carbocations are electron seeking reagents, chemists call them electrophiles. All Lewis acids, including protons, are electrophiles. By accepting an electron pair, a proton achieves the valence shell configuration of helium: carbocations achieve the valence shell configuration of Neon.
Stability: Carbocations are stabilised by
(i) + M effect
(ii) delocalisation of charge
(iii) Hyperconjugation
(iv) + 1 effect
General stability order: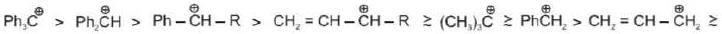
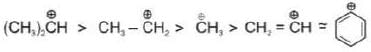
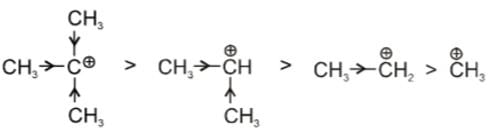 t-Butyl cafbocation has +I effect of three Me-groups and also Hyperconjugation effect which makes it most stable.
t-Butyl cafbocation has +I effect of three Me-groups and also Hyperconjugation effect which makes it most stable.
Example: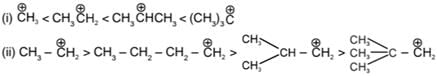
(due to resultant of inductive effect and hyperconjugation)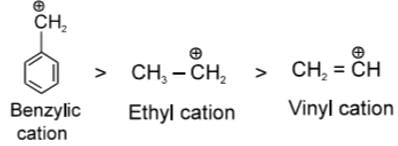 In Benzyl cation. Extensive Resonance is seen which stabilises
In Benzyl cation. Extensive Resonance is seen which stabilises 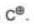
In Ethyl carbocation +I and Hyperconjugation of Me - group stabilizes carbocation.
In vinyl carbocation stability decreases rapidly smce carbon of (CH2) is sp2 hybridized which is slightly more electronegative hence acts as -I group w hich increases (+) charge density.
Stability:
Its stability can be determined with the help of Inductive effect, Hyperconjugation and Resonance effect.
Stability of Carbocation: Stability of carbocation can also be determined by Hyperconjugation (no bond Resonance).
(d) Carbenes (Divalent Carbon intermediates): There is a group of intermediates in which carbon forms only two bonds. These neutral divalent carbon species are called carbenes. Most carbenes are highly unstable that are capable of only fleeting existence. Soon after carbenes are formed, they usually react with another molecules.
Methods of preparation of carbene:
| Types of carbene | Singlet | Triplet  |
| Shape | Bent | Linear |
| Hybridisation | sp2 | sp |
| Nature o f reaction | stereospecific | None |
| State | Excited state | Ground state |
| Magnetic | Diamagnetic | Paramagnetic |
| Nature | Paired electrons | Diradical |
(e) Nitrenes: The nitrogen analog of carbenes are nitrenes. They are very much reactive since in them octet of N is incomplete. In nitrenes only one valencies of N are satisfied. (f) Benzyne: The benzene ring has one extra C - C π bond in benzyne
(f) Benzyne: The benzene ring has one extra C - C π bond in benzyne Clearly, we can see that the newly formed π bond cannot enter in resonance with other π orbitals of ring, since it is in perpendicular plane.
Clearly, we can see that the newly formed π bond cannot enter in resonance with other π orbitals of ring, since it is in perpendicular plane.
It is also important to note that hybridisation of each carbon involved in ‘Benzynic bond' is sp2 since the overlap between these sp2 hybrid orbitals is not so much effective.
Example: Compare the stability of the following carbocation:
(a)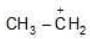
(b)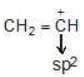
(c)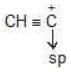
→ more s character
→ more electronegativity
→ +ve charge on more electronegative element is symbol of unstability.
Solution: a > b > c
Example: Compare the stability of the following compounds:
(a)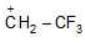
(b)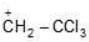
(c)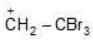
(d)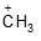
Solution: d > c > b > a
Being most electron attracting group it decreases the e- density from positively charged C-atom and decreases the charge density and makes the carbocation less stable.
Example: Compare the stability of the following carbocation:
(a)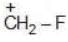
(b)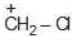
(c)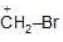
(d)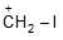
Solution: Due to greater size of Iodine, its L.P. will not be available for coordinate bond. Therefore L.P. would not stabilize carbocation.
In case of F due to its small size its lone pair can be easily coordinated to making it most stable.
a > b > c > d (Stability)
* By coordination the carbocation completes its octet and structure having complete octet of its atom is supposed to be most stable.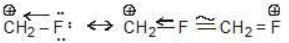
(Each atom has its full octet)
 Note: In Resonating Structure of at least one C gets sixtet of e-s and hence less stable than coordinated compound.
Note: In Resonating Structure of at least one C gets sixtet of e-s and hence less stable than coordinated compound.
Example: Compare the stabilities of the following carbocation:
(a)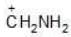
(b)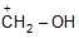
(c)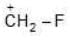
Solution: N, O, F belongs to same period
→ In period Electronegativity of the atom is deciding factor
→ F being most electronegative, holds its e- pair very firmly.
→ Its L.P. will not be easily available for coordination.
→ Stability by it will be minimum.
a > b > c
Example: Compare the following carbocation in order of their stability:
(a)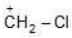
(b)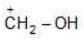
Solution: If periods of atoms which have to donate their electrons for coordination (for stability) is different then atomic size will be deciding factor. The atom whose size is greater will be unable to make its e- pair available for coordination.
b > a.
Example: Compare the stability of the following compounds:
(a)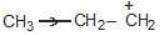
(b)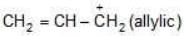
(c)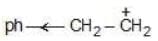
Solution:
→ more s-character
→ more e.n.
→ attracts e-
→ reduces stability
b > a > c
Carbanion:
1. It is a -ve charged species
2. It has octet of electrons.
3. Diamagnetic
Structure:
* If -ve charge is in Resonance then the hybridisation of carbanion is sp2 (Triangular planar shape).
* If -ve charge is not in Resonance then the hybridisation of carbanion is sp3(pyramidal).
Stability:
Its stability can be determined with the help of
(1) Inductive effect
(2) Resonance effect
Example:
(a)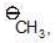
(b)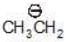
Solution:
a > b (stability)
* Stability of the carbanion is as follows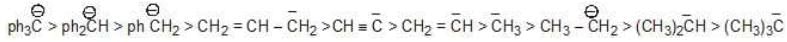
Example: Compare the stability of the following carbocation:
(a)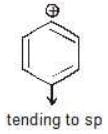
(b)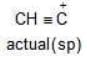
(c)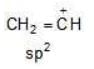
Solution: c > a > b
Example: Compare the stability of the following carbanion:
(a)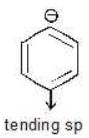
(b)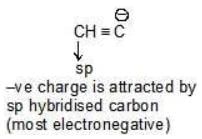
(c)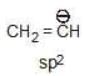
→ become more stable
Solution: b > a > c
Example: Compare the stability of the following carbanion:
(a)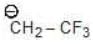
(b)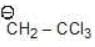
(c)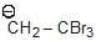
Solution: a > b > c
Example: Arrange the following anion in order of their stability:
(a) Cl-
(b) Br-
(c) F-
(d) I- (maximum size)
→ maximum dispersion of -ve charge
→ max stability
Solution: d > b > a > c
Example: Compare the stability of the following:
(a)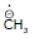
(b)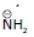
(c) 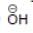
(d)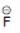
Solution: Same period element (C, N, O, F)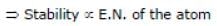
d > c > b > a
Example: Compare the acidic strength:
(a) HCl
(b) HF
(c) HBr
(d) HI
Solution: Acidic strength ≈ stability of the anion formed (conjugate base) As we know, I- > Br- > Cl- > F-
→ HI > HBr > HCl > HF
Example: Compare the Acidic strength of the following:
(a) NH3
(b) pH3
(c) AsH3
(d) SbH3
(e) BiH3
Solution: Anion formed from these acids are: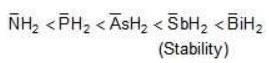 → acidic strength e > d > c > b > a
→ acidic strength e > d > c > b > a
Example: Compare the acidic strength of the following compounds:
CH4, NH3, H2O, HF
Solution: The conjugate base of the given acid is as follows: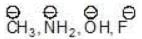 we have already proved that
we have already proved that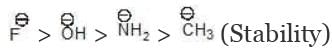 → HF > H2O > NH3 > CH4 (acidic strength)
→ HF > H2O > NH3 > CH4 (acidic strength)
Example: Compare the stability of the following carbanion:
(a)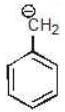
(b)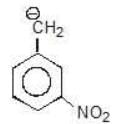
(c)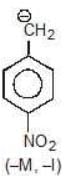
(d)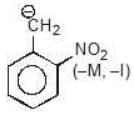
Solution: d > c > b > a
* M or -M is not distance dependent
Example: compare the stability of the following carbocation:
(a)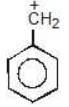
(b)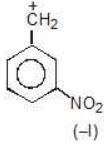
(c)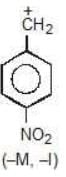
(d)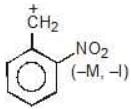
Solution: a > b > c > d
Example: Compare the stability of the following carbocation:
(a)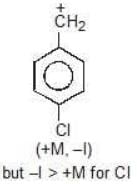
(b)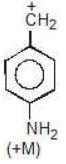
(c)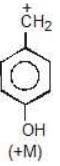
(d)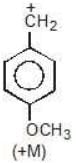
Solution: M (OH) > M (OCH3)
b > c > d > a
Example: Compare the stability of the following carbocation:
(a)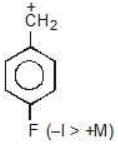
(b)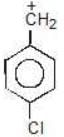
(c)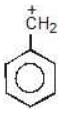
Solution: c > a > b
FAQs on Carbocation & Carbanions - JEE
| 1. What is a carbocation? |  |
| 2. What is a carbanion? |  |
| 3. How are carbocations formed? |  |
| 4. What are the properties of carbocations? |  |
| 5. How do carbocations and carbanions differ in terms of stability? |  |



















