Inductive and Mesomeric Effect - Class 11 PDF Download
INDUCTIVE EFFECT
In a covalent bond between two atoms having different electronegativities, the electron pair shifts towards the more electronegative atom resulting in the origin of small fractional charges on the constituent atoms. If one of the hydrogens of terminal carbon is replaced by electronegative atom X, then X will attract electrons from another carbon atom but this carbon with a slight electron deficiency acquires indirect electronegative character and withdraws electrons from adjacent carbon atoms which, in turn, also gets induced electronegativity and propagates the effect in the entire chain.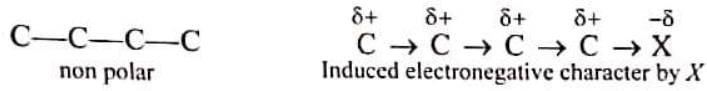 This induction of polarity in an otherwise nonpolar bond due to the presence of an electronegative element is known as inductive effect.
This induction of polarity in an otherwise nonpolar bond due to the presence of an electronegative element is known as inductive effect.
Thus , the displacement of shared electron pair in a sigma bond towards the more electronegative atom in a molecule is called as inductive effect. This effect is transmitted through the chain of σ bonds and diminishes with increasing chain length.
Inductive effect is thus
- A permanent effect
- The electrons never leave their original atomic orbital
- Operates through σ bonds
- Polarisation of electrons is always in single direction
- It is generally observed in saturated compounds
- its magnitude (i.e., electron withdrawing or donating power) decreases with increase in distance on the basis of inductive effect groups can be of two types:
-I group: The group which withdraws electrons is known as –I group and its effect is known As –I effect.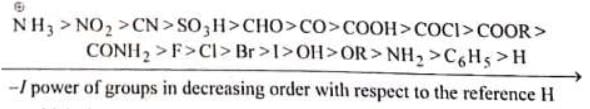 + I group: The group which donates or gives electrons is known as +I group and effect is as +I effect.
+ I group: The group which donates or gives electrons is known as +I group and effect is as +I effect.
(a) +l power of different type of alkyl groups: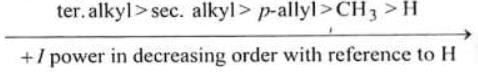 (b) +l power of same type of alkyl groups:
(b) +l power of same type of alkyl groups:
-l power ∝ number of C's in same type of alkyl group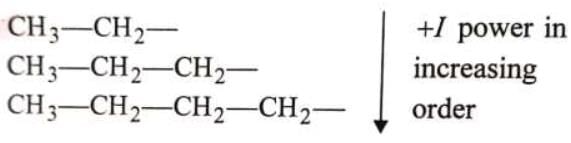
APPLICATIONS OF INDUCTIVE EFFECT
Amongst the various application of inductive effect, the two important application of immediate concern are :
(i) Determination of relative acid and base strength
(ii) Determination of relative stability of reactive intermediates roduced during a chemical reaction
Acid Strength and its Determination by Inductive Effect
Acid strength pertains to their extent of ionization when dissolved in a solvent (usually water). A stronger acid undergo grater extent of ionization while, a weaker acid undergo smaller extent of ionization when dissolved in water. Some acids are completely (100%) ionized when dissolved in water v/z HCI, HBr, HI, H2SO4 , HNO3 , HCIO4 , HIO4 etc. Therefore, strength of such acids are not usually compared in water, rather their strengths are compared in some other solvents (no the part of present discussion). Besides the above mentioned acids, a majority of remaining acids are only partially ionized in water, and that too with varying degree of ionization. It is these classes of acids, called weak acids whose strength can be compared with the help of inductive effect. Let us consider two weak monobasic acids to understand further the meaning of strength of acid.
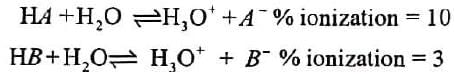
In the light of above information, it can be deduced that HA is stronger acid than HB , although both are very weak acids in absolute sense. In present section, our objective is to compare the relative strength of organic acid eg, carboxylic acids (ROOH), alcohols (ROH) , phenol(PhOH), sulphonic acid ( RSO3 ) etc. TO compare the strength of these weak acids, we devise a generalized mechanism of ionization of a weak acid and look for the role of groups present in the acid in the process of ionization Iomzanon of acid-a generalized mechanism
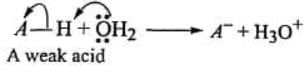 In the above ionization process, water is acting as a base (nucleophile) while, H is acting as an electrophile. During ionization the shared pair of electrons of A-H bond is take away by A. From this ionization mechanism following can be concluded.
In the above ionization process, water is acting as a base (nucleophile) while, H is acting as an electrophile. During ionization the shared pair of electrons of A-H bond is take away by A. From this ionization mechanism following can be concluded.
- An electron withdrawing A can easily accommodate the shared pair of electrons after ionization and therefore, facilitate the ionization process, Hence, an electron withdrawng group increases the acid strength.
- An electron donating A will be reluctant in holding the shared pair of electron (as A-) after ionization and therefore oppose the ionization process. Hence, and electron donating A decreases the acid strength.
Unsubstituted, aliphatic alcohols (ROH) are all weaker acids than water, rationalize. Aliphatic alcohols and water have certain similarity in their bonding as
H — O — H, R — O — H
Ionization of these two acids can be shown as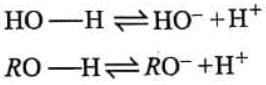 Since, alkyl (R-) group has electron donating (+I) effect, it opposes the ionization of O - H bond and decreases the acid strength.
Since, alkyl (R-) group has electron donating (+I) effect, it opposes the ionization of O - H bond and decreases the acid strength.
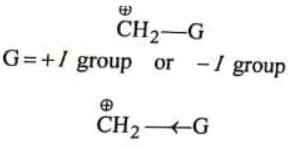
Where Case I: Suppose G is +I group, then
(i) Magnitude of positive charge will be decreased +I group, and
(ii) More is the +I power of the group, less will be magnitude of positive charge.Hence, magnitude of positive charge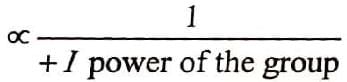
Case II: Supose G is –I group, then : 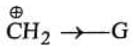
(i) Magnitude of positive charge will be increased by –I group, and
(ii) More is the _-I power of the group, greater will be magnitude of the positive charge. Hence magnitude of positive charge ∝ -I power of the group
Magnitude of negative charge 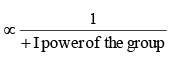
Magnitude of negative charge ∝ +I + I power of the group
STABILITY OF CATION AND ANION
Rule I: Less is the magnitude of charge, more will be stability of charge species. Stability of similar charge species (i.e., stability between cations or stability between anions) can be compared in those cases where:
(i) Charge is present on the same atoms in all species, adn
(ii) Hybridisation of atoms bering the charge should be same i the all species.
For example: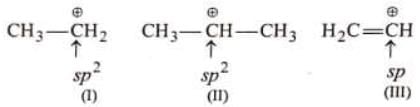 Stability of action I and II can be compared by rule-I because positive charge in (I) and (II) is present on same atom (i.e., carbon ) and hybridisation of C is same in both cases.
Stability of action I and II can be compared by rule-I because positive charge in (I) and (II) is present on same atom (i.e., carbon ) and hybridisation of C is same in both cases.
Stability of (I) and (III) cannot be compared by rule-I because hybridisation of 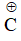 in (I) and (III) is
in (I) and (III) is
different.
Rule 2: for maximum stability: Positive charge should be present on electropositive atom in cation or negative should be present on electronegative atom in anion.
And (III) or (II) and (III) can be compared by this rule.
Stability of alkyl carbocation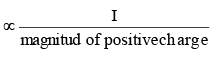
Magnitude of positive charge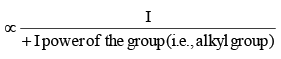
Stability of alkyl carbocation ∝ +I power of the group present on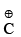 Thus tertiary alkyl carbocation is more stable than secondary which is more stable than primary carbocation
Thus tertiary alkyl carbocation is more stable than secondary which is more stable than primary carbocation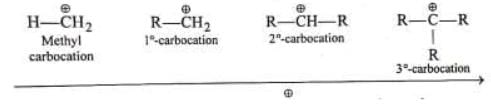 (i) Number of +I groups on C is in increasing order
(i) Number of +I groups on C is in increasing order
(ii) +I power on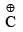 is in increasing order
is in increasing order
(iii) Magnitude of positive charge is in decreasing order
(iv) Stability is in increasing order
Stability of alkyl carbanion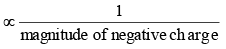
Magnitude of negative charge ∝ + I power of the group (i.e., alkyl group)
Stability of alkyl carbonion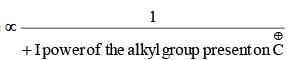
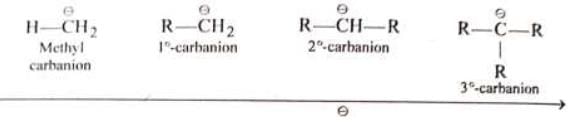
(i) Number of alkyl groups on C is in increasing order
(ii) +I power on C is in increasing order
(iii) negative charge on is in increasing order
is in increasing order
(iv) Stability is in decreasing order Hence alkyl carbocation is always more stable than vinyl carbocation
Hence alkyl carbocation is always more stable than vinyl carbocation
 Negative charge is present on more electronegative carbon acetylenic carbanionis more stable than vinylic carbanion which is more stable than alkyl-carbanion.
Negative charge is present on more electronegative carbon acetylenic carbanionis more stable than vinylic carbanion which is more stable than alkyl-carbanion.
Acid strength ∝ Ka
Acid strength ∝ concentration of acid anion
Acid strength ∝ stability of acid anion, i.e., conjugate base of an acid
Example: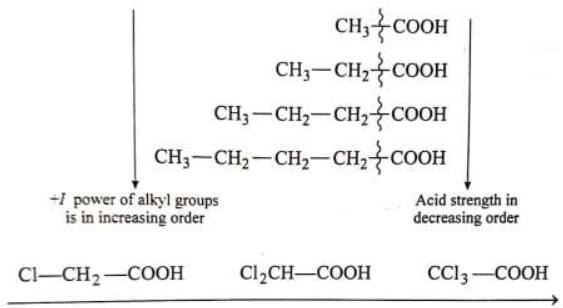 -I power is in increasing order, acid strength is in increasing order
-I power is in increasing order, acid strength is in increasing order
(i) Inductive effect: It is an effect in which permanent polarisation arises due to partial displacement of s-electrons along carbon chain or partial displacement of sigma-bonded electrons toward more electronegative atom in carbon chain.
Magnitude of partial positive charge:
d1 > d2 > d3 = d- (net charge remains constant in a molecule having inductive effect)
Inductive effect: It is a permanent effect.
if X i.e more electronegative
(After carbon No. 3 the effect disappears)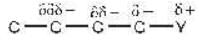 ( I effect of Y)
( I effect of Y)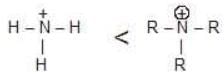 (- I effect order)
(- I effect order)
* O- < O < O (-I effect order)
- It is a permanent effect.
- It is caused due to electronegativity difference.
- It operates via s bonded electron.
- It is distance dependent effect.
- As distance increases, its effect decreases.
- It can be neglected after third carbon.
- It has a destablising effect.
- It is divided into 2 parts. (On the basis of electronegativity w.r.t. hydrogen atom)
(1) + I effect
(2) - I effect
If any atom or group having electronegativity greater than that of hydrogen, then it is considered as - I effect and vice-versa.
+I effect - I effect: difference between +I and –I effect
| +I effect | - I effect |
| (i) e- releasing group | (i) e- accepting group |
| (ii) EN less than H | (ii) EN greater than H |
| (iii)Those groups which are showing + I effect, disperses partial - ve charge on the Cchain. | (iii) Those groups showing -I effect disperse + ve charge on the C-chain. |
Example:
CH3 - CH2 - CI(-I of CI)
CH3 - CH = CH2(-I of -CH = CH2 & +I of -CH3)
CH3 - CH2 - C ≡ CH (-I of -C ≡ CH & +I of -CH2 -CH3)
I - CI
+I - I
Order of -I effect showing group
Order of + I effect showing group
RESONANCE
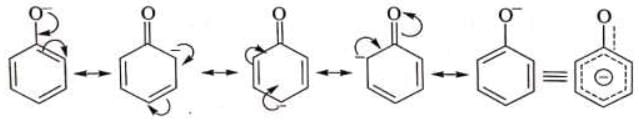
When a single Lewis dot structure is unable to explain all the properties of a commond, two or more structures, called the canonical forms on resonating structures, are drawn to explain all the properties of that compound. Then the actual structure of the compound is said to be in between these canonical forms and is called the resonance hybrid of these forms. This phenomenon is called resonance, e.g. benzene is a resonance hybrid of following two canonical forms.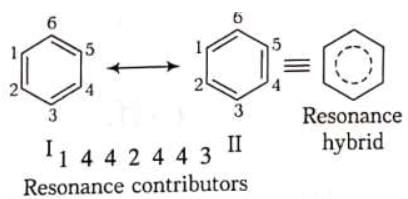
Resonance contributors are shown with a double-headed arrow separating them. It also indicates that the actual structure lies somewhere between the structure of the resonance contributors. They do not depict any real electron distribution and are imaginary. The resonance hybrid is always stable than any of its canonical forms,
e.g.
(i) CO2 is said to have following canonical forms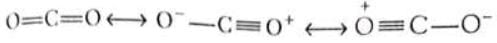 (ii) Benzene may be represented by following structures
(ii) Benzene may be represented by following structures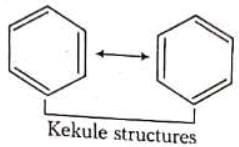
In both the above examples the actual structure of CO2 or benzene is more stable than either of the structures described.
Conditions for resonance
- The arrangement of atoms are identical or almost same in every formula
- The energy content of all the canonical forms is nearly the same
- Each canonical form has the same number forms is unpaired electrons.
- All the atoms in a molecule taking part resonance should be present in same plane.
Stability of canonical forms
(i) Among all the canonical form s, the form without charges will be the most stable one.
(ii) Among the charged forms, the structure in which maximum number of covalent bonds resent is the most stable. The structure with less charge separation of opposite charge and more dispersal of charge is more stable than the others. Such as
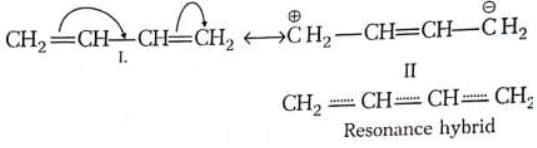 In the given example, I and II are canonical structure. The I structure is more stable due to presence of maximum number of covalent bonds and structure II is less stable due to charge separation, therefore structure I contributes more towards resonance hybrid.
In the given example, I and II are canonical structure. The I structure is more stable due to presence of maximum number of covalent bonds and structure II is less stable due to charge separation, therefore structure I contributes more towards resonance hybrid.
e.g.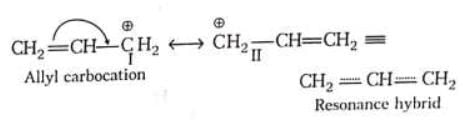
Structure I and II both are identical hence, have same stability and contribute towards resonance hybrid.
RESONANCE ENERGY
The resonance hybrid is always more stable than any one of its canonical form. Heat of hydrogenation of resonance hybrid is abnormally high.
e.g. the observed heat of hydrogenation of 6 6
C6H6 (=-49.8 kcal/mol) is greater than the calculated
Value (=-85.8 kcal/mol) of the heat of hydrogenation of its most stable structure.
The difference in heat of hydrogenation, i.e.
Heat of hydrogenation – Heat of hydrogenation
(observed) (calculated)
Is called the resonance energy, which is assumed to be the energy gained when molecule acquires resonance hybrid formula.
TYPES OF RESONATING STRUCTURES
There are two types of resonating structures
(i) Isovalent resonating structures: The structures in which the number of bonds in different contributing structures are same, e.g.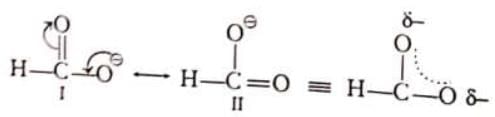 (ii) Heterovalent resonating structures: The structures in which the number of bonds in different contributing structures are different.
(ii) Heterovalent resonating structures: The structures in which the number of bonds in different contributing structures are different.
Example: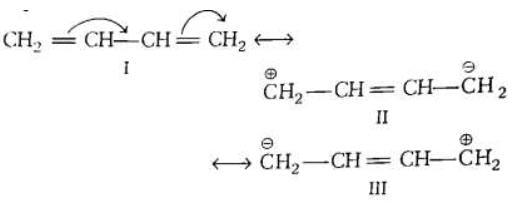 APPLICATIONS
APPLICATIONS
The various applications of resonance are:
- Determines the stability of some free radicals, carbocations and carbanions.
- Determines the low reactivity of aryl and vinyl hlide.
- Determines the acidic nature of carboxylic acid and explain the greater stability and less nucleophilicity of carboxylate ion (RCOO-).
Stabilisation of carboxylate ion occurs by delocalisation of its negative charge.
Greater resonance stabilisation is possible in carboxylate anion when compound with the resonance stabilisation of undissociated carboxylic acid molecule.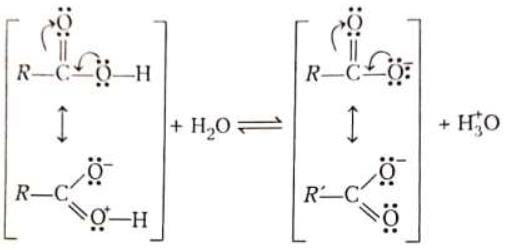
(iv) Determines the basic character of aromatic amines. The basic strength of aromatic amines is increased by using +R effect substituent as they disperse the positive charge on the N atom and stabilise the anilinium ion while substituent with –R effect decrease the basic strength of aromatic amines as they destabilise aniliniumions.
Decreasing order of basic strength.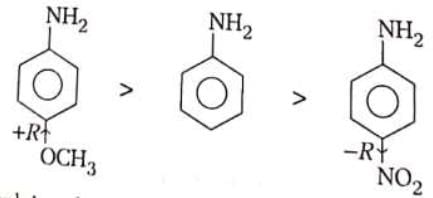
Examples: Draw the major resonance contributor of the following
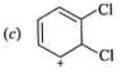
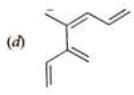
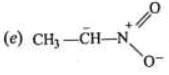
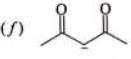

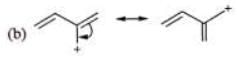
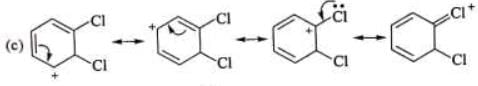
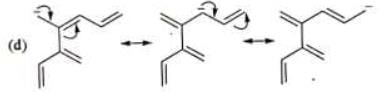
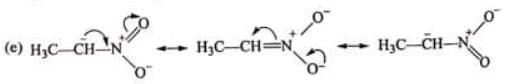
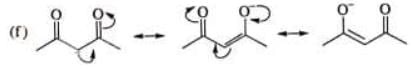
NOTE - Although phenoxide ion ( C6H5O\ O−) has more resonance structures than carboxylate ion ( RCOO−) carboxylic acid is stronger acid than phenol, justify.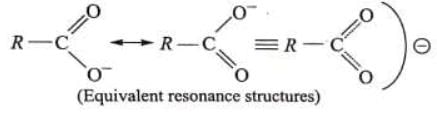
Due to above reasons, carboxylate ion is more stable than phenoxide ion inspite of having less number of resonance structures and carboxylic acid is stronger acid than phenol.
MESOMERIC EFFECT (M-EFFECT)
It is defined as “polarity produced in a molecule due to conugation”. It is a permanent effect.
CONJUGATION
The corrugation or conjugative bond signifies the partial n -bond in organic compounds It is seen in those cases where n-bond is present along with another rc-bond, charge, odd electron, or lone pair of electron For conjugation it is must that these entities are separated by only a single bond.
The result of conjugation is seen as improper bond length and unpredictable extra stability in the molecule. Benzene is the best example of this partial π-bonding.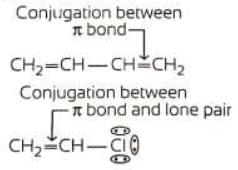
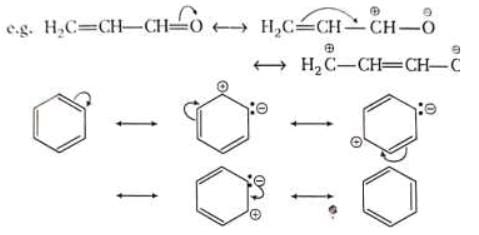
Due to mesomeric effect terminal carbon is almost as positive as the first carbon. This is quite different from inductive effect due to which charge decreases as one moves away from the source.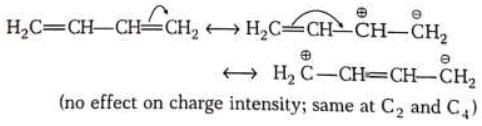
TYPE OF MESOMERIC EFFECT
On the basis electron withdrawing or electron donating nature of conjugation, this effect can be of following two types
(i) Positive mesomeric effect (+M): A group or atom is said to show + M-effect when the direction of electron displacement is away from it.
Such groups have lone-pair of electrons, and release the pair for conjugation with an attached unsaturated (conjugated) system.
In simple words, this effect extends the degree of delocalisation of the ( -)ve charge imparted to the molecule due to the process of conjugation. Examples executing +M-effect are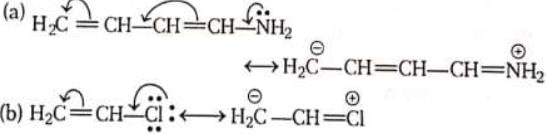
The +M-effect of halogen atom in vinyl halides and aryl halides explain their low reactivity.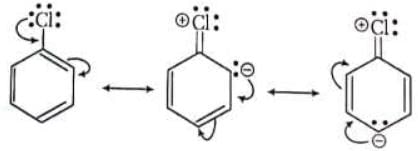
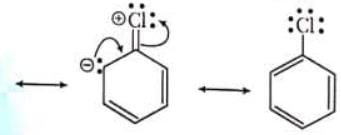
Decreasing order for + M power of groups are O - > NH2>OH>OR>NHAc> alkyl groups (CH3 > 1o > 2o > 3o ) > phenyl groups
(ii) Negative mesomeric effect (-M): A group or atom is said to have –M- effect when the direction of electron displacement is towards such group (but away from conjugate system). Such groups are electron withdrawing.
E.g. Due to electron withdrawing nature of oxygen atom following structures are possible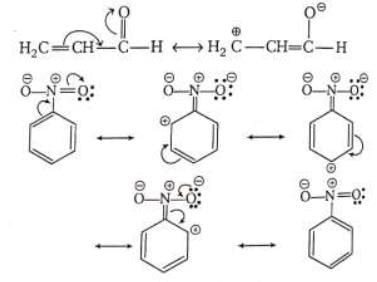
Decreasing order for (-M) power of groups are
NH3 > NO2 > CX3 > CN > SO3H > CHO > CO > COOH > COCI > COOR > CONH2
Example: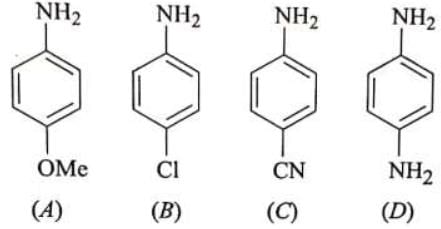
➢ The increasing order of basic strength is : C<B<A<D\
D is strongest base. It is stronger than A because in D , less electronegative nitrogen is involved in electron donation by resonance (+R) while, in A, more electronegative oxygen is involved in electron donation. Between B and C,B has electron withdrawing-I effect but electron denoting resonance effect. On the other hand—CN is electron withdrawing group by both inductive and resonance effect, hence, weaker base than B.
Bond Strength: CT3 > CD3 > CH3 (+I of T > D > H)
Ques. Why carbon - hydrogen bond is longer than C - T bond
Ans. As the mass increases, vibration decreases as a result of which the heavier isotope will be more closer to the C-atom for a longer time. Therefore C - T bond is stronger. C - T > C - D > C - H which implies that C - H bond has longest bond length.
Mesomeric effect in phenol ( M effect):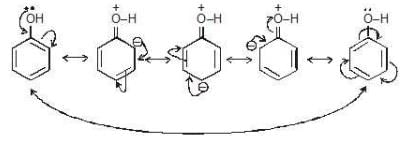
+M effect in aniline: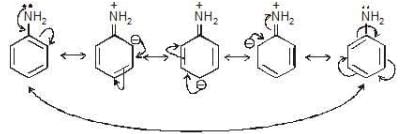 If the movement of e-s is towards ring → ( M effect)
If the movement of e-s is towards ring → ( M effect)
→ This effect increases the electron density over benzene ring.
* -M effect in Benzaldehyde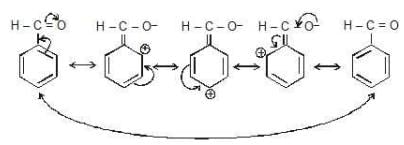
Example: Identify the compound showing M or -M separately
(a)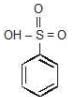
(b)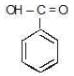
(c)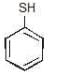
Solution: (a) (-M) (b) (-M) (c) +M
- M group increases electron density of ring while - M decreases the electron density of benzene ring.
- if NO2 is present on the ortho or para position then along with its -I effect, It will also show -M effect.
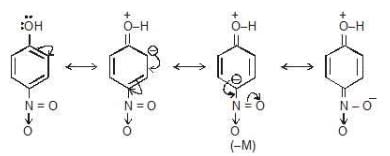
- Above compound have M of -OH and -M of NO2 group.
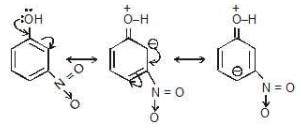 as we can easily see that -NO2 at meta position is not attracting e-density towards itself and that's why it will not show -M effect at m-position.
as we can easily see that -NO2 at meta position is not attracting e-density towards itself and that's why it will not show -M effect at m-position.
COMPARISON OF INDUCTIVE, HYPERCONJUGATION AND
RESONANCE EFFECTS
Inductive Effect is a σ - σ interaction and acts through strong sigma bonds.
Resonance/Mesomeric Effect is a ti-ti interaction and acts through weak pi bonds.
Hyperconjugation is a σ - π interaction and acts through a strong sigma and a weak pi bond. Therefore, the order of importance is:
Resonance > Hyperconjugation > Inductive
Example:
Arrange the following in decreasing order of basic strength.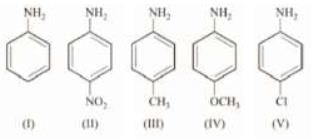 Solution:
Solution: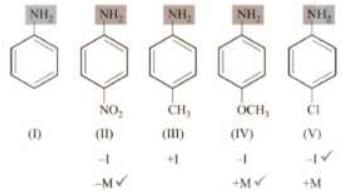 Therefore, order of basic strength is: iv > iii > i > v > ii Let’s also discuss the stability of anilimium ions corresponding to (ii) and (iv).
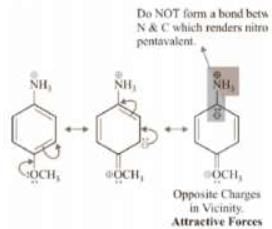
Therefore, order of basic strength is: iv > iii > i > v > ii Let’s also discuss the stability of anilimium ions corresponding to (ii) and (iv).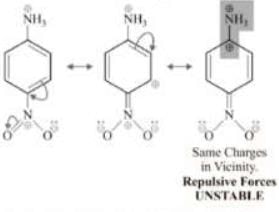
Example: Mark the order of bond lengths in the given molecule.
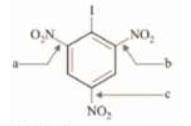 Solution: - I , - NO2, are bulky groups and is case of steric inhibition of resonance. Therefore, the - N02 groups ortho to - I are out of conjugation while the - N02 group para to - I will be in conjugation with the benzene ring. Therefore, bonds ‘a ’ and ‘b ’ will always have single bond character while ‘c’ has double bond character. Therefore:
Solution: - I , - NO2, are bulky groups and is case of steric inhibition of resonance. Therefore, the - N02 groups ortho to - I are out of conjugation while the - N02 group para to - I will be in conjugation with the benzene ring. Therefore, bonds ‘a ’ and ‘b ’ will always have single bond character while ‘c’ has double bond character. Therefore:
c < a = b
Note:
STERIC INHIBITION OF RESONANCE (SIR)
When both the ortho positions of a bulky functional group are occupied by bulky substituents, all the three groups are out of plane of the benzene ring.
Example: Mark the number of α-C and α-H in the given compounds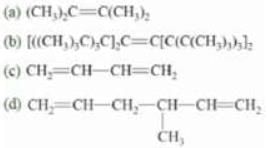
Solution: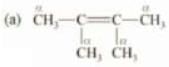
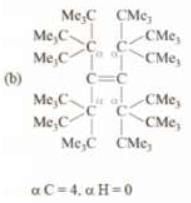

αC = 1, αH = 1 but since α C is sp2 hybridized, therefore, it won’t participate in hyperconjugation. Therefore, α H = 0 that will participate in hyperconjugation.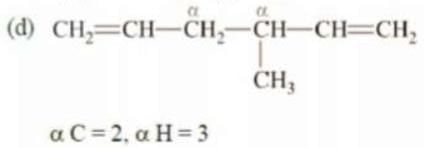
Example: Which of the following structures is more stable?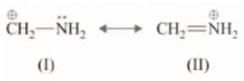
Solution: II is more stable as all the octets are complete.
Example: Which of the following is more stable in the following pairs?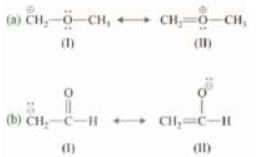
Solution:
(a) ln II, all octets are complete. Therefore. II is more stable.
(b) l and II are tied on octets and number of n bonds but negative charge is more stable on more electronegative atom. Hence, II is more stable.
Note: Series of +1 and -I groups in order of their strength -I Series (EWG)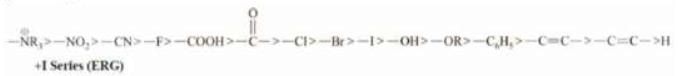
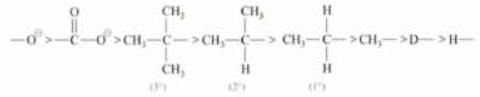
Note: Order of acidic strength
RSO3H > RCOOH > H2CO3 > PhOH > CH3OH > H2O > ROH > HC = CH > NH3 > CH4
FAQs on Inductive and Mesomeric Effect - Class 11
| 1. What is the inductive effect? |  |
| 2. What is the mesomeric effect? |  |
| 3. How does the inductive effect influence the acidity of organic compounds? |  |
| 4. What are the factors that affect the strength of the inductive effect? |  |
| 5. How does the mesomeric effect impact the stability of resonance structures? |  |














