INTRODUCTION OF MATTER - Class 11 PDF Download
INTRODUCTION
Anything that exhibits inertia is known as matter. The quantity of matter is its mass. e.g. chalk table.
In simple language,
Anything which has mass and occupies space is called matter.
Matter can exist in three physical states viz. solid, liquid and gas.
The constituent particles of matter in these three states can be represented as shown in Figure.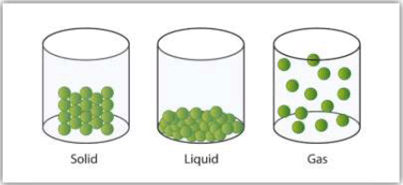
A Representation of the Solid, Liquid,
and Gas States
- In solids, these particles are held very close to each other in an orderly fashion and there is not much freedom of movement.
- In liquids, the particles are close to each other but they can move around.
- In gases, the particles are far apart as compared to those present in solid or liquid states and their movement is easy and fast.
Because of such arrangement of particles, different states of matter exhibit the following characteristics:
(i) Solids have definite volume and definite shape.
(ii) Liquids have definite volume but not the definite shape. They take the shape of the container in which they are placed.
(iii) Gases have neither definite volume nor definite shape. They completely occupy the container in which they are placed.
These three states of matter are inter-convertible by changing the conditions of temperature and pressure.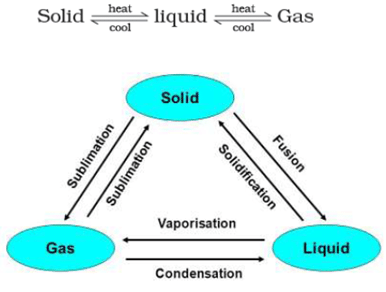
CLASSIFICATION OF MATTER (AT MACROSCOPIC / BULK LEVEL)
This classification of matter is based upon chemical composition of various substances. According to this matter can be further divided into two types, pure substance and mixture. Mixtures are also of two types, homogenous mixtures and heterogeneous mixtures.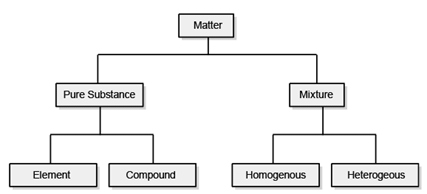
Elements : The primary stuff present in all the substance is known as element, whose smallest unit is known as atom.
Sodium, copper, silver, hydrogen, oxygen etc. are some examples of elements. They all contain atoms of one type. However, the atoms of different elements are different in nature.
- Total 118 elements are known till date of which 92 are naturally occurring elements rest are results of artificial transmutation. There are 88 metals, 18 non metals and 6 metalloids.

Compound : A non-elemental pure substance is called a compound in which more than one atom of elements are linked by chemical bonds formed due to chemical reaction. The resulting molecule is an electrically neutral particle of constant continuous composition.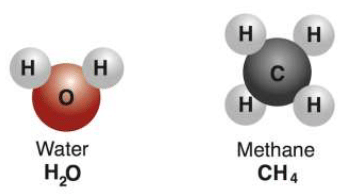
MIXTURE : Mixtures are the aggregate of more than one type of pure substance whose chemical identity remains maintained even in mixtures. Their constituent ratio may vary unlike compound.
For example :
(i) sugar + water = sugar syrup,
(ii) Gun-powder 75 % KNO3 10% Sulphur + 15% carbon
There are two types of mixture
(a) Homogeneous
(b) Heterogenous
(a) Homogeneous mixtures are those whose composition for each part remains constant. For example, aqueous and gaseous solution.
(b) Heterogeneous mixtures are those whose composition may vary for each and every part. For example, soil, concrete mixtures.
HOMOGENEOUS MIXTURES VERSUS HETEROGENEOUS MIXTURES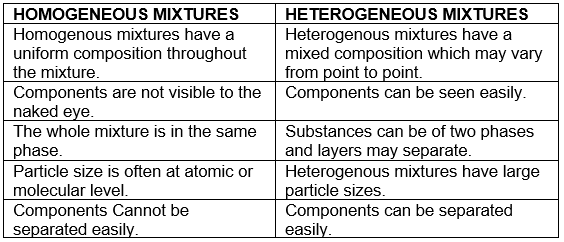
COMPOUND VERSUS MIXTURE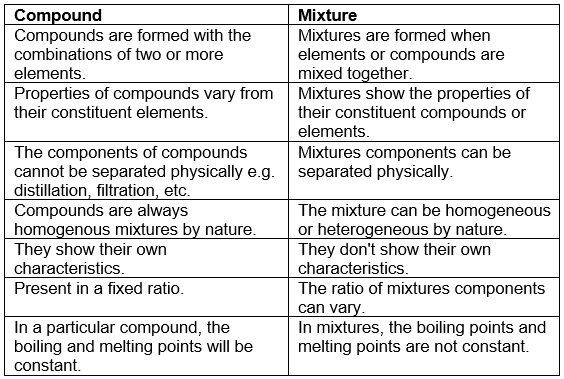
Try Yourself!
Q.1. Which one of the following is not an element?
(a) Diamond
(b) Graphite
(c) Silica
(d) Ozone
Ans. (c)
Solution. Silica (SiO2) is a compound and not an element.
Q.2. The most abundant element on the earth’s crust is
(a) Iron
(b) Aluminium
(C) Oxygen
(d) Nitrogen
Ans. (c)
Solution. The most abundant element on earth’s crust is Oxygen.
Q.3. Which one of the following is not mixture?
(a) Iodized table salt
(b) Gasoline
(c) Liquefied Petroleum Gas (L.P.G)
(d) Plaster of Paris (POP)
Ans. (d)
Solution. Plaster of Paris is a compound (CaSO4).
Q.4. Which one if the following is not a metalloid?
(a) Arsenic
(b) Antimony
(c) Germanium
(d) Bismuth
Ans. (c)
Solution. Germanium is not a metalloid.













