Environmental Pollution (Part - 4) | Environment for UPSC CSE PDF Download
What is Acid Rain?
Acid rain is a rain or any other form of precipitation that is unusually acidic, meaning that it has elevated hydrogen ions. It can have harmful effects on plants, aquatic animals, and infrastructure.
Types of Acid Deposition
1. Wet Deposition
- If the air's acid chemicals are blown into areas where the weather is wet, the acids can fall to the ground in the form of rain, snow, fog, or mist.
- As this acidic water flows over and through the ground, it affects various plants and animals.
- The strength of the effects depends on several factors, including how acidic the water is; the chemistry and buffering capacity of the soils involved; and the types of fish, trees, and other living things that rely on the water.
- Precipitation removes gases and particles from the atmosphere by two processes:
(i) rain-out, which incorporates particles into cloud drops that fall to the ground, and
(ii) washout occurs when materials below the cloud are swept down by rain or snow it falls.
2. Dry Deposition
- In areas where the weather is dry, the acid chemicals may become incorporated into dust or smoke and fall to the ground through dry deposition, sticking to the ground, buildings, vegetation, cars, etc.
- Dry deposited gases and particles can be washed from these surfaces by rainstorms, through runoff.
- This runoff water makes the resulting mixture more acidic.
- About half of the acidity in the atmosphere falls back to earth through dry deposition.
The pH scale
- The pH scale is a measure of how acidic or basic (alkaline) a solution is.
- It ranges from 0 to 14. A pH of 7 is neutral.
- A pH less than 7 is acidic, and a pH greater than 7 is basic.
- It was devised in 1909, and it is a logarithmic index for the hydrogen ion concentration in an aqueous solution.
- pH values decrease as hydrogen ion levels increases.
- A pH 4 is ten times more acidic than a pH 5, and a hundred times more acidic than a solution with pH 6.
- The pH range is usually given as 0 to 14, and lower and higher values are theoretically possible.
1. Sources of compounds causing acid rain
(a) Sulphur
(i) Natural sources
- seas and oceans,
- volcanic eruptions,
- Biological processes in the soil, e.g., Decomposition of organic matter.
(ii) Man-made sources
- burning of coal (60% of SO2) and
- petroleum products (30% of SO2), and
- The smelting of metal sulfide ores to obtain pure metals.
- Industrial production of Sulfuric acid in metallurgical, chemical and fertilizer industries.
(b) Nitrogen
(i) Natural sources
- lightening,
- volcanic eruption, and
- Biological activity.
(ii) Anthropogenic sources
- Forest fires
- Combustion of oil, coal, and gas
(c) Formic acid
- Biomass burning due to forest fires causes the emission of formic acid (HCOOH) and formaldehyde (HCHO) into the atmosphere.
- Large fraction formaldehyde gets photo-oxidation and forms formic acid in the atmosphere.
These are three main compounds that cause acidification of rain in the atmosphere.
(d) Other Acids
- Chlorine
- Phosphoric acid
- Hydrochloric acid (smokestacks).
- Carbon monoxide and carbon dioxide (automobiles). These become carbonic acid.
Does it occur only in industrial areas alone?
SOX and NOX that create Acid Rain are often transported to distances far away from their points of origin by the wind so that the adverse effects of pollution are also experienced at a place remote from the place of genesis. The problem is further compounded as the environmental damage caused by acid rain is not uniform, but is area-specific.
2. Common characteristics of acid rain areas
Areas which are prone to acid-rain attacks have some common characteristics
- They are concentrated in the industrialized belt of the northern hemisphere.
- They are often upland and/or mountainous areas, which are well-watered by rain and snow.
- Due to the abundance of water, they possess numerous lakes and streams and have more land covered with vegetation.
- Being upland, they often have thin soils and glaciated bedrock.
➤ World Wide
- Many parts of Scandinavia, Canada, the North and Northeast United States and Northern Europe (particularly West Germany and upland Britain) share these features. Across the Atlantic, several acid rain hot spots include Nova Scotia, Southern Ontario and Quebec in Canada, the Adirondack Mountains in New York, Great Smoky Mountains, parts of Wisconsin, Minnesota, and the Colorado Rockies of the US.
➤ In India
- In India, the first report of acid rain came from Bombay in 1974. Instances of acid rain are being reported from metropolitan cities. In India, the annual SO2 emission has almost doubled in the last decade due to increased fossil fuel consumption. Lowering soil pH is reported from north-eastern India, coastal Karnataka and Kerala, parts of Orissa, West Bengal and Bihar.
➤ Indicators
- Lichens serve as good bio-indicators for air pollution. In the variety of pH around 6.0, several animals are important food items for the fish decline. These include the freshwater shrimp, crayfish, snails and some small mussels.
3. Chemistry of Acid Rain
Six basic steps are involved in the formation of acid rain
- The atmosphere receives oxides of sulfur and nitrogen from natural and human-made sources.
- Some of these oxides fall back directly to the ground as dry deposition, either close to the place of origin or some distance away.
- Sunlight stimulates the formation of photo-oxidants (such as ozone) in the atmosphere.
- These photo-oxidants interact with the oxides of sulfur and nitrogen to produce H2SO4 and HNO3 by oxidation.
- The oxides are of sulfur and nitrogen, photo-oxidants, and other gases (like NH3)
- Acid rain containing ions of sulfate, nitrate, ammonium and hydrogen falls like a wet deposition.
Difference between normally and anthropogenically acidified lakes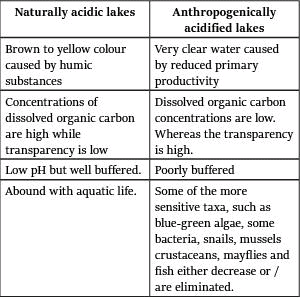
4. Impact Of Acid Rain
(i) Soil
- The exchange between hydrogen ions and the nutrient cations like potassium and magnesium in the soil cause leaching of the nutrients, making the soil infertile.
- This is accompanied by a decrease in the respiration of soil organisms.
- An increase in ammonia in the soil due to a decrease in other nutrients decreases the decomposition rate.
- The nitrate level of the soil is also found to decrease.
- The impact of acid rain on the soil is less in India; because Indian soils are mostly alkaline, with good buffering ability.
(ii) Vegetation
Acid rains affect trees and undergrowth in the forest in several ways, causing reduced growth or abnormal growth:
The typical growth-decreasing symptoms are
- Discolouration and loss of foliar biomass
- Loss of feeder-root biomass, especially in conifers
- Premature senescence (ageing) of older needles in conifers
- Increase in susceptibility of damage to the secondary root and foliar pathogens
- Death of herbaceous vegetation beneath affected trees
- Prodigious production of lichens on affected trees.
- Death of affected trees.
(iii) Microorganisms
- pH determines the proliferation of any microbial species in a particular environment and the rate at which it can produce.
- Most bacteria and protozoa's optimum pH is near neutrality; most fungi prefer an acidic environment; most blue-green bacteria prefer an alkaline environment.
- So after a long run of acid rain, microbial species in the soil and water shift from bacteria-bound to fungi-bound and cause an imbalance in the microflora.
- This causes a delay in the decomposition of soil organic material and an increase in fungal disease in aquatic life and forests.
(iv) Wildlife
The effects of acid rain on wildlife are not very obvious and are, therefore, difficult to document. Nevertheless, several direct and indirect effects of acid rain on wildlife populations' productivity and survival have been reported.
- Acid rain can directly affect the eggs and tadpoles of frogs and salamanders that breed in small forest ponds.
- It has been postulated that acid rain can indirectly affect wildlife by allowing metals bound on soils and sediments to be released into the aquatic environment, where toxic substances may be ingested by animals, like birds, that feed in such an environment.
- Other indirect effects of acid rain on wildlife are loss or alteration of food and habitat resources.
(v) Humans
Acid rain affects human health is several ways.
- The obvious ones are bad smells, reduced visibility; irritation of the skin, eyes and the respiratory tract.
- Some direct effects include chronic bronchitis, pulmonary emphysema and cancer.
- Some indirect effects include food poisoning vis a vis drinking water and food.
- An increase in toxic heavy metals like manganese, copper, cadmium, and aluminium also contributes to human health's detrimental effects.
Do you know?
- Bonsai-i.e., tailored or human-made miniature or dwarfed living trees that have been prevented from reaching their normal size-are grown in pots and kept in greenhouses, drawing rooms, etc. The Japanese first perfected this technique.
- Bamboos are trees without the main trunk but with a cluster of culms from the underground rhizome. These culms are unbranched, with distinct nodes and internodes that give them a jointed appearance.
- Trees reduce oxides of carbon in the air, can also fix atmospheric nitrogen, disintegrate waste and act as sinks of pollution
- Sometimes seeds of a plant a reformed without fertilization. This phenomenon is called "agamospermy," a kind of parthenogenesis. A fruit that matures without seed formation is called "parthenocarpic fruit."
- Beverage plants are those plants which yield beverages or drinks-nonalcoholic or alcoholic-that are palatable and refreshing. Nonalcoholic beverages usually contain caffeine, an alkaloid, which has stimulating and refreshing qualities. Alcoholic beverages are those that contain one or more hydroxyl (–OH) groups; e.g., ethanol
(vi) Acid rain damage on Materials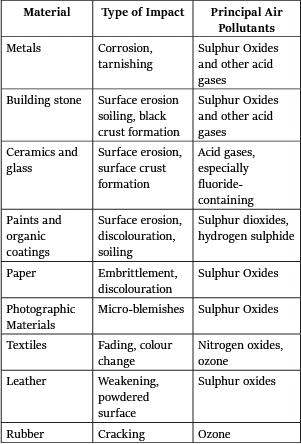
(vii) Socio-economic impacts of acid rain
- The adverse impact of acid rain on farming and fishing leads to the deterioration of life quality indices like GNP and per capita income, especially in the predominantly agricultural and developing countries like India.
5. Trigger Effect of Acid Rain on Pollutants
A low pH of the rainwater and subsequently increased acidity in the environment can trigger or aggravate certain harmful pollutants.
(i) Mercury
- Methyl mercury and related short chain alkyl mercurial compounds are most dangerous to humans, as they accumulate in edible fish tissue.
- Although acid deposition may not increase methyl mercury production, it may increase the partitioning of methyl mercury into the water column.
- The use of lime has helped in reducing the mercury levels in fish.
(ii) Aluminium
- Acidified waters are known to leach substantial amounts of aluminium from watersheds.
- Even at relatively low levels, aluminium has been implicated in dialysis dementia, a central nervous system disorder, which may be toxic to individuals with impaired kidney function.
(iii) Cadmium
- Cadmium can enter the drinking water supply through corrosion of galvanized pipe or copper-zinc through corrosion of galvanized piper or from the copper-zinc solder used in the distribution systems.
- A decrease in water pH from 6.5 to 4.5 can result in a fivefold increase in cadmium and cause renal tubular damage.
(iv) Lead
- Foetuses and infants are highly susceptible to drinking water lead contamination.
- High blood lead levels in children (>30 mg/Ml) are believed to induce biochemical and neurophysiological dysfunction.
- However, lower than normal blood levels of lead can cause mental deficiencies and behavioural problems.
(v) Asbestos
- Acidic waters can release asbestos in natural rock.
6. Control Measures
Reducing or eliminating the sources of pollution by
- Buffering- the practice of adding a neutralizing agent to the acidified water to increase the pH is one of the important control measures. Usually, lime in the form of calcium oxide and calcium carbonate is used.
- Reducing the emission of SO2 from power stations by burning less fossil fuel, using alternate energy sources like tidal, wind, hydropower etc.,
- using low sulphur fuel;
- desulphurization
- decreasing emission of NOx from power stations and
- Modification of engines.
- Emissions of SOx can be controlled by
(i) Converting to sulphuric acid.
(ii) Converting it to elemental sulphur.
(iii) Neutralizing it and using it in the manufacture of other products.
Categorization of Industrial Sectors
- The Ministry of Environment, Forest and Climate Change (MoEFCC) has developed the criteria of categorization of industrial sectors, Red, Orange, Green and White categories based on the Pollution Index which is a function of the emissions (air pollutants), effluents (water pollutants), hazardous wastes generated and consumption of resources. The Pollution Index PI of any industrial sector is a number from 0 to 100, and the increasing value of PI denotes the increasing degree of pollution load from the industrial sector.
- "Re-categorization of industries based on their pollution load is a scientific exercise. The old system of categorization created problems for many industries and was not reflecting the industries' pollution. The new categories will remove this lacuna and will give a clear picture to everyone. "The new category of White industries that is practically non-polluting will not require Environmental Clearance (EC) and Consent and will help get finance from lending institutions. No Red category of industries shall normally be permitted in the ecologically fragile area / protected area.
|
95 videos|230 docs|52 tests
|
FAQs on Environmental Pollution (Part - 4) - Environment for UPSC CSE
| 1. What causes acid rain? |  |
| 2. How does acid rain affect the environment? |  |
| 3. What are the effects of acid rain on human health? |  |
| 4. Can acid rain be prevented or controlled? |  |
| 5. Which areas are most affected by acid rain? |  |





















