NCERT Summary: Summary of Chemistry - 3 | Science & Technology for UPSC CSE PDF Download
2. Classifications
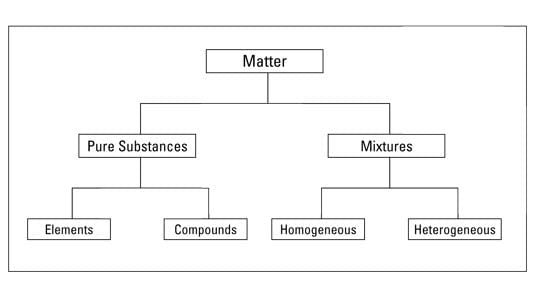
(a) Pure Substance and Mixture
- A pure substance is one that contains one kind of materials throughout its body. A substance cannot be separated into other kinds of matter by any physical process.
- Mixtures are constituted by more than one kind of pure form of matter, known as a substance. Mixtures can be separated into pure substances using appropriate separation techniques like filtration, sublimation, decantation, chromatography, crystallization, etc.
(b) Homogeneous and Heterogeneous Substance
- A substance is said to be homogeneous if it has one and the same composition and properties in all its parts. On the other hand, if the composition and properties are not identical throughout the body the substance is heterogeneous. A pure substance must be homogeneous.
(c) Elements and Compounds
Pure substance are classified into elements and compounds:
- Elements: An element is a form of matter that cannot be broken down by chemical reactions into simpler substances. Robert Boyle was the first scientist to use the term element in 1661. Elements can be normally divided into metals, non-metals and metalloids.
- Compound: A compound is a substance composed of two or more different types of elements, chemically combined in a fixed proportion. Properties of a compound are different from its constituent elements.
Symbols: The symbol is an abbreviation for the full name of an element. In many cases the initial capital letter of the common name of element is used as abbreviation for it.
» H stands for Hydrogen, N for Nitrogen, etc. Two letters are used in cases of two or more elements having the same initial letter. A second prominent letter ( small) from its name is added to the initial letter.
» Al stands for Aluminium, Cl stands for chlorine, etc. In some cases the symbols are derived by taking letter or letters from the Latin name of the element. Cu stands for Copper ( Latin name Cuprum), Au stands for Gold ( Latin name Aurum), etc.
➢ Representation Using Symbols
- The symbol represents one atom and naturally stands for a perfectly definite amount of the element concerned. Every substance is an aggregate of its molecules, and the symbolic representation of a molecule of the substance is called its formula.
- The number of atoms per molecule of the element is known as the atomicity of the molecule. If the molecule of an element contains one atom, then the molecule is represented by the symbol only, i.e., in such a case symbol also represents the formula.
- Valency: The number of chemical substances, except the element themselves, are composed of two or more of these elementary materials combined together.
The valency of an element is the combining capacity of an atom of the element and is measured by the number of hydrogen atoms with which it can be combined.
- Hydrogen is chosen as the standard of reference because the combining capacity of hydrogen is least. Though the combining capacity of an atom of the element is by and large fixed, valency may vary, some elements exhibit different valencies. The highest valency known being 0, the valencies range between 0 and eight. Helium, argon, etc., the so-called inert gases have no combining capacity and hence they are regarded as zero-valent element. A valency is always a whole number.
- Compounds too like elements are represented by molecular formula. To build up the formula of a compound the symbols of the constituent elements are written side by side and the number of atoms of each is indicated by putting numerals to the lower right of the symbols. But the subscript one is not written in formula.
3. Solution
- A solution is a homogeneous mixture of two or more substances. The major component of a solution is called the solvent, and the minor, the solute. Lemonade, soda water etc. are all examples of solutions. We can also have solid solutions (alloys) and gaseous solutions (air).
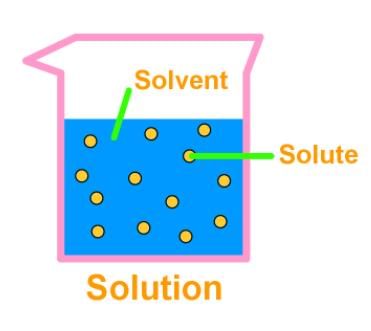
- The particles of a solution are smaller than 1 nm (10-9 metre) in diameter. So, they cannot be seen by naked eyes. The solute particles cannot be separated from the mixture by the process of filtration. The solute particles do not settle down when left undisturbed, that is, a solution is stable.
- The concentration of a solution is the amount of solute present per unit volume or per unit mass of the solution/ solvent.
- Materials that are insoluble in a solvent and have particles that are visible to naked eyes form a suspension. A suspension is a heterogeneous mixture.
4. Alloys
- Alloys are homogeneous mixtures of metals and cannot be separated into their components by physical methods. But still, an alloy is considered as a mixture because it shows the properties of its constituents and can have variable composition.
Example: Brass is a mixture of approximately 30% zinc and 70% copper. - Non-homogeneous systems, in which solids are dispersed in liquids, are called suspensions. A suspension is a heterogeneous mixture in which the solute particles do not dissolve but remain suspended throughout the bulk of the medium. Particles of a suspension are visible to the naked eye.
- Colloids are heterogeneous mixtures in which the particle size is too small to be seen with the naked eye, but is big enough to scatter light. Colloids are useful in industry and daily life. The particles are called the dispersed phase and the medium in which they are distributed is called the dispersion medium.
5. Metals and Non-Metals
Elements can be normally divided into metals, non-metals and metalloids.
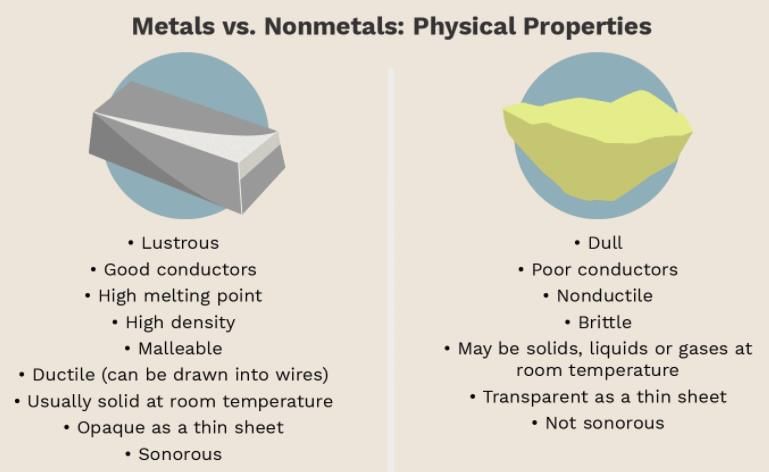
Metals usually show some or all of the following properties:
- They have a lustre (shine).
Exception: Mercury, though a metal is liquid. - They have silvery-grey or golden yellow colour.
- They conduct heat and electricity. Silver is the best while copper stands second.
- They are ductile (can be drawn into wires). Gold is the most ductile metal.
- They are malleable (can be hammered into thin sheets).
Exception: Metals like antimony and bismuth are brittle. - They are sonorous (make a ringing sound when hit).
- Metals have high melting points.
Exception: Gallium and Caesium have very low melting points. - Metals can form positive ions by losing electrons to non-metals. In electrolysis, metals get deposited at the negative electrode(cathode).
- Metals combine with oxygen to form basic oxides. Aluminium oxide and zinc oxide show the properties of both basic as well as acidic oxides. These oxides are known as amphoteric oxides.
- Different metals show different reactivities towards oxygen. Metals such as potassium and sodium react so vigorously that they catch fire if kept in the open. Hence, to protect them and to prevent accidental fires, they are kept immersed in kerosene oil.
- Different metals have different reactivities with water and dilute acids. Metals above hydrogen in the Activity series can displace hydrogen from dilute acids and form salts.
- Metals occur in nature as free elements or in the form of their compounds. The extraction of metals from their ores and then refining them for use is known as metallurgy.
- The surface of some metals, such as iron, is corroded when they are exposed to moist air for a long period of time. This phenomenon is known as corrosion.
Organic Chemistry
- Organic chemistry is that branch of chemistry which deals with the study of compounds of carbon with hydrogen (hydrocarbons), and their derivatives. Presently about five million organic compounds are known.
- Organic compounds were found to contain mainly hydrogen and carbon. Therefore, organic chemistry is defined as the study of hydrocarbons and their derivatives. Most atoms are only capable of forming small molecules. However one or two can form larger molecules.
- By far and away the best atom for making large molecules with is Carbon. Carbon can make molecules that have tens, hundreds, thousands even millions of atoms! The huge number of possible combinations means that there are more carbon compounds that those of all the other elements put together!
- A single carbon atom is capable of combining with up to four other atoms. We say it has a valency of 4. Sometimes a carbon atom will combine with fewer atoms. The Carbon atom is one of the few that will combine with itself. In other words, carbon combines with other carbon atoms. This means that Carbon atoms can form chains and rings onto which other atoms can be attached. This leads to a huge number of different compounds.
- Organic Chemistry is essentially the chemistry of Carbon. Carbon compounds are classified according to how the Carbon atoms are arranged and what other groups of atoms are attached.
➢ Hydrocarbons
The simplest Organic compounds are made up of only Carbon and Hydrogen atoms only. Even these run into thousands! Compounds of Carbon and Hydrogen only are called Hydrocarbons.
1. Alkanes
In the alkanes, all four of the Carbon valency bonds are taken up with links to different atoms. These types of bonds are called single bonds and are generally stable and resistant to attack by other chemicals. Alkanes contain the maximum number of Hydrogen atoms possible. They are said to be saturated.
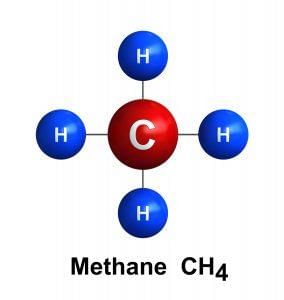
The simplest Hydrocarbon is:
- Methane: CH4, this is the simplest member of a series of hydrocarbons. Each successive member of the series has one more carbon atom than the preceding member.
- Ethane: C2H6
- Propane (heating fuel): C3H8
- Butane (lighter / camping fuel): C4H10
- Pentane: C5H12
- Hexane: C6H14
- Polythene is a very large alkane with millions of atoms in a single molecule. Apart from being flammable, alkanes are stable compounds found underground.
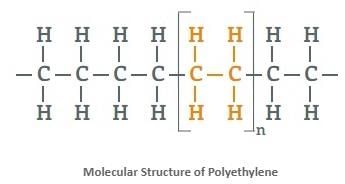
2. Alkenes
Another series of compounds is called the alkenes. These have a general formula: CnH2n. These compounds are named in a similar manner to the alkanes except that the suffix is one. Alkenes have fewer hydrogen atoms than the alkanes. The extra valencies leftover occur as double bonds between a pair of carbon atoms. The double bonds are more reactive than single bonds making the alkenes chemically more reactive.
The simplest alkenes are listed below:
- Ethene (used as an industrial starter chemical): C2H4
- Propene: C3H6
- Butene: C4H8
- Pentene: C5H10
- Hexene: C6H12
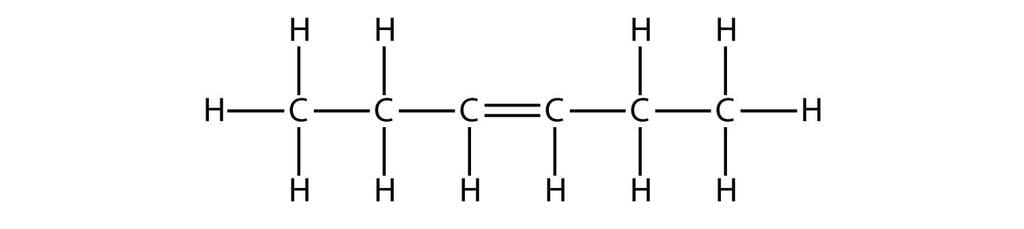 The Structural Formula for Hexene
The Structural Formula for Hexene
3. Alkynes
A third series are the alkynes.
These have the following formula: CnH2n-2
These highly reactive substances have many industrial uses. Again the naming of these compounds is similar to the alkanes except that the suffix is -yne. Alkynes have two carbon atoms joined by a triple bond. This is highly reactive making these compounds unstable.
Examples of alkynes are:
- Ethyne - Better known as acetylene which is used for welding underwater: C2H2
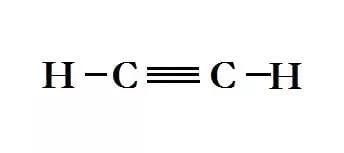 Structural Formula for Ethyne
Structural Formula for Ethyne - Propyne: C3H4
- Butyne: C4H6
- Pentyne: C5H8
- Hexyne: C6H10
Alkanes, alkenes and alkynes all contain carbon atoms in linear chains. When rings are combined with chains, the number of hydrocarbons is virtually infinite. There are also hydrocarbons arranged in rings.
Some examples follow:
- Cyclohexane - A saturated hydrocarbon with the atoms arranged in a hexagonal ring: C6H12
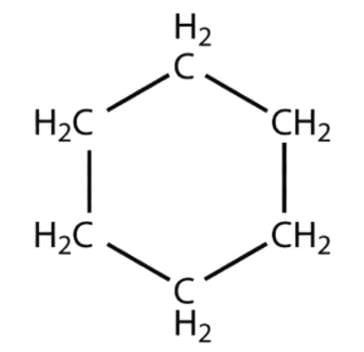 Cyclohexane
Cyclohexane - Benzene - An industrial solvent. The Benzene Ring is one of the most important structures in organic chemistry. In reality, its alternate double and single bonds are “spread around” the ring so that the molecule is symmetrical: C6H6
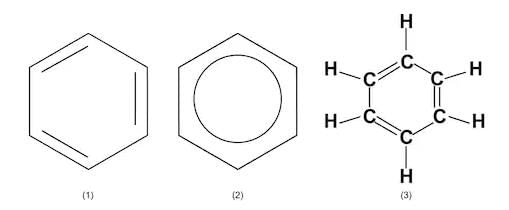 Benzene
Benzene - Toluene - An important solvent and starter chemical: C7H8
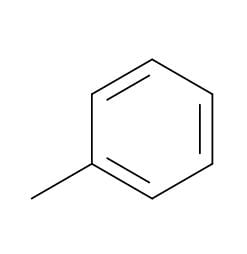 Toluene
Toluene
- Naphthalene - Used in mothballs. This can be depicted as two fused Benzene Rings: C10H8
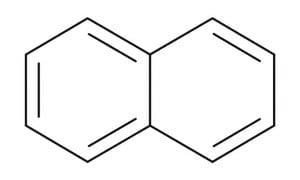 Naphthalene
Naphthalene
➢ Carbon, Hydrogen and Oxygen
When Oxygen atoms are added, the variety of compounds grows enormously.
Here are some examples where each molecule has a single functional group:
- Alcohols: Alcohols have the OH (hydroxyl) group in the molecule. A group of atoms that gives an organic series its distinctive character is called a functional group. These have a general formula: CnH2n+1OH.
Examples:
(i) Methanol (wood alcohol) CH3OH
(ii) Ethanol(drinking alcohol) C2H5OH
(iii) Phenol(carbolic acid - used as a disinfectant) C6H5OH - Ethers (Ethers have an O atom attached to two hydrocarbon chains) (CnH2n+1)2O.
Examples:
(i) Dimethyl Ether(a gas) (CH3)2O
(ii) Diethyl Ether (a liquid used as an anaesthetic) (C2H5)2O - Ketones (Ketones have a CO group attached to two hydrocarbon chains). These have a general formula: (CnH2n+1)2CO.
Example: Dimethyl Ketone (Also known as acetone: Nail varnish remover), CH3COCH3 - Aldehydes (Aldehydes have a CHO group attached to a hydrocarbon chain). These have a general formula: CnH2n+1CHO.
Example:
(i) Formaldehyde (preservative in labs)- HCHO
(ii) Acetaldehyde- CH3CHO - Fatty Acids (Fatty Acids contain the CO2H (or COOH) group attached to a hydrocarbon chain or ring). These have a general formula: CnH2n+1CO2H.
Example:
(i) Formic Acid(in ant bites and stinging nettles)- HCO2H.
(ii) Acetic Acid( vinegar)- CH3CO2H.
(iii) Butyric Acid( the rancid butter smell)- C2H5CO2H. - Esters (Esters are similar to Fatty Acids except that the H in the COOH group is another hydrocarbon chain. They are usually very sweet-smelling liquids used in perfumes). These have a general formula: RCO2R’( R and R’ are Hydrocarbon chain or rings).
Examples: Methyl Methoate (the essence of pear drops) - CH3CO2CH3.
It is possible to have two or more functional groups on a molecule. These can be the same group (as in Oxalic Acid - a poison found in rhubarb leaves - which has two fatty acid groups) or different (as in Hydroxyethanoic Acid - which has a hydroxyl group and a fatty acid group): Oxalic Acid- (COOH)2, Hydroxyethanoic Acid- CH2OHCOOH.
The most famous compounds containing Carbon, Hydrogen and Oxygen are the Carbohydrates. An example is the common sugar, Sucrose (C12H22O11).
➢
Isomerism
- An interesting phenomenon with organic molecules is called isomerism. Let us look at two compounds introduced earlier. Dimethyl Ether: (CH3)2O and Ethanol: C2H5OH. The first is a gas which will knock you out if inhaled. The second is common alcohol drunk in spirits. Both compounds contain 2 Carbon atoms, 6 Hydrogen atoms and 1 Oxygen atom.
- Even though the atoms are the same, they are arranged differently. This yields two different compounds with the same number of atoms. These compounds are isomers and the phenomenon is called Isomerism. Isomerism increases the number of Organic compounds. The more carbon atoms in a compound, the more ways of arranging the atoms and the larger number of isomers.
➢ Compounds Containing Nitrogen
- Adding Nitrogen: Many, very important organic compounds contain Nitrogen. This produces more series of compounds.
(a) Amines (Amines have one or more of the Hydrogen atoms in Ammonia (NH3) replaced by a Hydrocarbon chain or ring).
These have a general formula: CnH2n+1NH2.
Examples:
(i) Methylamine (a pungent, water-soluble gas)- CH3NH2
(ii) Cyanides (Cyanides have the CN group)
» These have a general formula: CnH2n+1CN
Example: Methyl Cyanide- CH3CN
(iii) Amino Acids (Amino Acids have two functional groups: the amine (HN2) group and the fatty acid (COOH) group
» These have a general formula: CnH2nNH2COOH
Example: Glycine (the simplest amino acid)- CH2NH2COOH
(iv) A famous compound containing Nitrogen is TrinitroToluene (C6H2CH3(NO)3) - usually abbreviated to TNT). This is an artificially made explosive.
The vast majority of organic compounds contain Carbon, Hydrogen, Oxygen and Nitrogen. Other types of atoms can be included to form even more compounds. These can contain atoms like Phosphorus, Sulphur (Example: Thiamine,), Chlorine (Example: Chlorophyll-CHCl3, Dichloro Diphenyl Trichloro Methane – DDT C14H9Cl15) and Iron (Example: Haemoglobin).
Periodic Classification of Elements
- The grouping of elements with similar properties together and the separation of elements with dissimilar properties is known as classification of elements. The table, which classifies elements on the basis of their properties, is called the periodic table. Döbereiner grouped the elements into triads and Newlands gave the Law of Octaves. Mendeléev arranged the elements in increasing order of their atomic masses and according to their chemical properties.
- Dobereiner’s Triads arranged elements in an increasing order of atomic mass, in groups of three. The atomic mass of the middle element was the arithmetic mean of the other two elements of the triad.
- Newland’s law of octaves states that on arranging elements in increasing order of their atomic mass, the eighth element resembles the first in physical and chemical properties, just like the eighth note on a musical scale resembles the first note.
- According to Mendeleev’s periodic law, the physical and chemical properties of elements are periodic functions of their atomic mass. Mendeleev corrected the atomic masses of a few elements on the basis of their positions in the periodic table. Mendeléev even predicted the existence of some yet to be discovered elements on the basis of gaps in his Periodic Table.
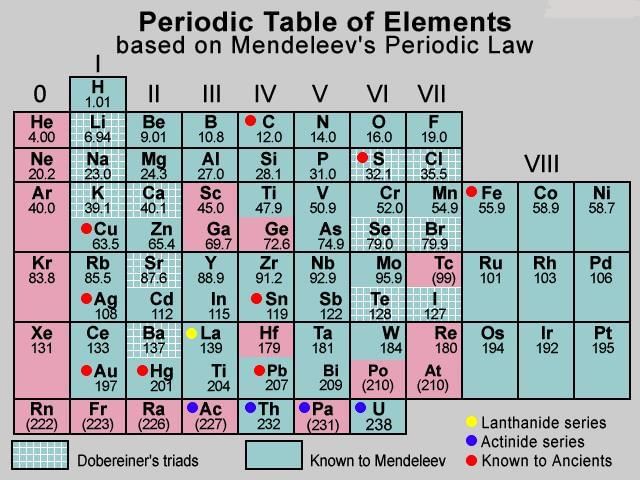
- Mendeléev’s Periodic Table contains vertical columns called ‘groups’ and horizontal rows called ‘periods’. While developing the Periodic Table, there were a few instances where Mendeléev had to place an element with a slightly greater atomic mass before an element with a slightly lower atomic mass. The sequence was inverted so that elements with similar properties could be grouped together.
- Mendeleev’s table could not assign a proper position to hydrogen or to the lanthanides and actinides and isotopes. Isotopes of all elements posed a challenge to Mendeleev’s Periodic Law. Another problem was that the atomic masses do not increase in a regular manner in going from one element to the next. So it was not possible to predict how many elements could be discovered between two elements — especially when we consider the heavier elements.
- In 1913, Henry Moseley showed that the atomic number of an element is a more fundamental property than its atomic mass. Accordingly, Mendeléev’s Periodic Law was modified and atomic number was adopted as the basis of Modern Periodic Table and the Modern Periodic Law.
- The vertical columns are called groups, while the horizontal rows are called periods. The noble gases are on the extreme right of the table and on the table’s extreme left, are the alkali metals. Transition elements are placed in the B subgroups in the middle of the table. The inner transition elements - lanthanides and actinides, are placed in two separate series at the bottom of the periodic table.
- Group number is number of electrons in the valence shell. Elements having the same valence number, are grouped together. The number of shells present in the atom gives period number.
- Atomic size: The term atomic size refers to the radius of an atom. The atomic size may be visualised as the distance between the centre of the nucleus and the outermost shell of an isolated atom.
|
90 videos|490 docs|209 tests
|
FAQs on NCERT Summary: Summary of Chemistry - 3 - Science & Technology for UPSC CSE
| 1. What is organic chemistry? |  |
| 2. What is periodic classification of elements? |  |
| 3. What are some key features of the periodic table? |  |
| 4. How are elements classified into different blocks in the periodic table? |  |
| 5. What is the importance of periodic classification of elements? |  |