Collision Theory - Class 11 PDF Download
Need for the theory?
- Whether a reaction is feasible or not?
- How much time will be required for the completion of the reaction?
- What are the deciding factors for making these reactions effective?
Collision theory
"Collision theory stated that the reactant atom/molecules undergo collisions to give products"
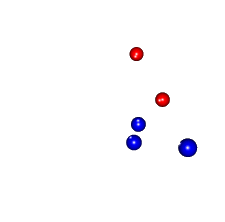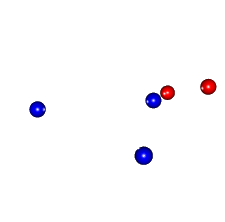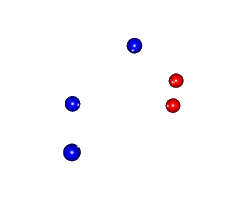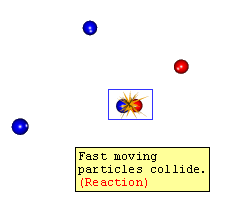
- If the reactant atoms/molecules are placed in a closed container
- Due to their random motion, many collisions take place
- Which results in the completion of the reaction with the formation of a product
Note:
Not every collision gives product only a few of them actually undergo changes.
which was due to improper orientation and not sufficient energy.
Factors affecting effective collision
- Orientation factor-: the one which results in the production of maximum no. of products.
- Energy factor-: minimum requirement of energy in order to produce a product.
- Temperature factor-: higher temperature is preferred.
- Catalyst presence-: helps to reduce the time take in the formation of the product.
➢
Orientation Factor
There are infinite ways for the atoms to collide but only a few of them are able to complete the reaction.
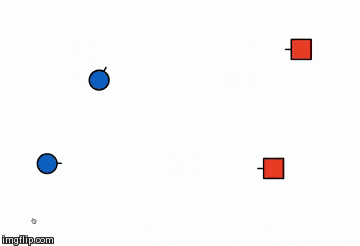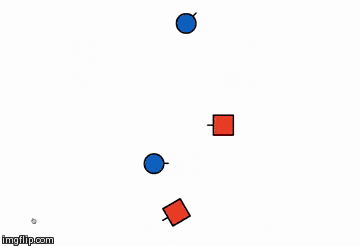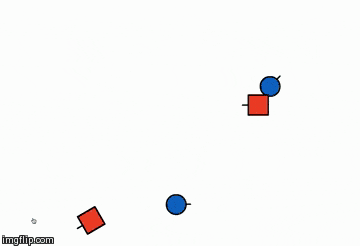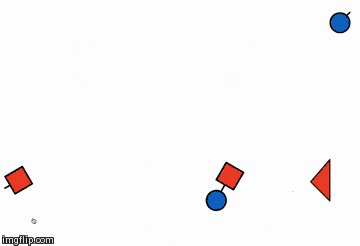 The illustration below is of the same reaction but in a different orientation,
The illustration below is of the same reaction but in a different orientation,
explaining how orientation plays an important role in the success of the reaction
 Orientation 1
Orientation 1
 Orientation 2
Orientation 2
- I.e. considering the product point of view 'Orientation 2' will be preferred because in a single collision more no of products are formed
➢Energy Factor
For the success of any reaction, there is a minimum amount of energy that should be possessed by the reactants.
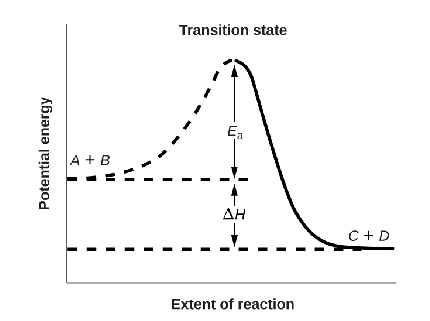 Energy Barrier Diagram
Energy Barrier Diagram
- A+B reactants having energy Er which is very much less than the required threshold energy
- The energy required to reach threshold energy is known as activation energy.
- As the reactants reach this point they tend to form an unstable complex, which releases energy to form the product C+D.
- This drop in energy from Ea to Ep is the release of this exothermic reaction
 |
Download the notes
Collision Theory
|
Download as PDF |
➢Temperature
As the temperature is increased, it tends to increase the energy of reactants and which results in more collisions, and hence the rate of reaction is increased.
It is found that on every increase of 10 ̊C, the reaction rate is increased by two or three times.
➢Catalyst
Due to the addition of a catalyst, there is a drop in activation energy which will result in the achievement of the complex state earlier.




















