Revision Notes: Stereochemistry of Organic Molecules | Organic Chemistry PDF Download
Points to be Remembered
1. Isomerism :
Compounds having identical molecular formula but having different structural formulas are known as isomers. The phenomenon is known as isomerism.
2. Classification of Isomerism : Isomerism is classified in the following tabular form :
Chain Isomerism : If the isomerism arises due to the isomers having different chain length.
Example - n-Pentane and neo-Pentane.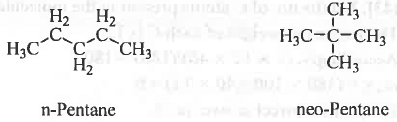
- Positional Isomerism : if the isomerism occurs due to the isomers having different positions of the functional groups.
Example - 1-Propanol and 2-Propanol.
- Functional Isomerism : If the isomerism occurs due to the isomers having different functional groups.
Example - Dimethyl ether and 1 -Butanol.
- Tautomerism : Isomerism where isomers changes reversibly into differing in the nature of functional group and remain in dynamic equilibrium is called tautomerism. Two tautomers differs only in the position of atoms not in the position of electrons. For showing tautomerization, the presence of at least one acidic a-H is the necessary condition.
- Conditions for the Stability of enol form : In general, the keto form is energetically more stable than the enol form. But, the % of enol form can be higher than the % of keto form due to the mainly following two reasons :
A. Enol form can be stabilised through the formation of intra-molecular H-bond.
B. Enol form can also get stabilised through resonance.
Example - Acetyl acetone (acac) can have two tautomeric forms - keto form and enol form.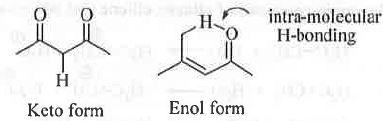
- Metamerism : It is a special type of structural isomerism applicable for multivalent atoms like O, N bearing two dissimilar alkyl groups.
Example - Diethyl ether and Methyl propyl ether.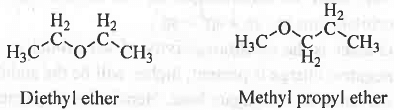
- Optical Isomerism : Here, we will check .whether a given compound is optically active or, in-active. There are four symmetry elements which can be present in a molecule. These are - 1. Plane of symmetry(σ); 2. Simple axis of symmetry (Cn) ; 3. Centre of inversion (i) ; 4. Alternating axis of symmetry (Sn). Cn is not related to the optical activity of a compound. But, if any of the rest three symmetry elements are present in the molecule then the molecule will become optically inactive.
Example - meso-Tartaric acid contains plane of symmetry. That's why it is optically inactive.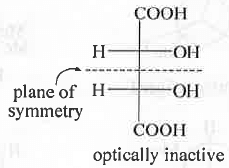
Geometrical Isomerism : It is classified into cis and tram isomers. An alkene of type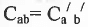 will show geometrical isomerism if and only if
will show geometrical isomerism if and only if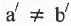 and
and 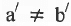 but
but 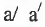 and
and 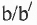 may be same or, different.
may be same or, different.
3. Optical Activity of a Compound :
I. Compounds may be optically active due to the presence of: a. chiral centre ; b. chiral axis ; c. chiral plane and d. helical chirality.
Compound 1 contains chiral axis. That's why it is optically active. Compound 2 contains chiral plane. That's why it is also optically active. Compound 3 possess helical Chirality. That's why is is optically active. Hence, the correct match is : a-ii, b-iii and c-iv.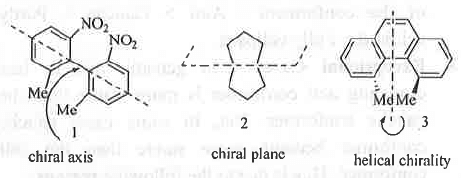
II. Compounds can be optically inactive due to by both the internal and external compensation
- Internal Compensation : This is judged by the presence of symmetry elements in the molecule. Cn is not related to the optical activity of a compound. But, if any of the rest three symmetry elements (σ, Sn and i) are present in the molecule then the molecule will become optically inactive. A brief description of the above symmetry elements are given below :
- Simple Axis of Symmetry (Cn) : It is an imaginary axis drawn through the molecule so that if the molecule rotates through an angle of 360°/n, another molecule which is indistinguishable from the original one results. A molecule can have more than one type of Cn axis with different values of n. Cn axis with highest value of’n' is called principal axis. Optical activity of a compound is independent on whether Cn is present or, absent.Example - Water molecule contains C2 axis.
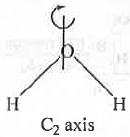
Plane of Symmetry (σ) : It is an imaginary plane drawn through the molecule which divide the molecule into two equal half such that one half is the mirror image of the other half. It can be of three types - Horizontal plane of symmetry (σh), Vertical plane of symmetry (σV) and Diagonal plane of symmetry (σd). If it is present in a molecule, the molecule will become optically inactive in spite of the chiral centre present in the molecule.
Example - Water molecule also contains σ plane.
- Centre of Symmetry (i): It is a point present within a molecule such that if a straight line is drawn from any part of the molecule through that point and extended an equal distance by a straight line on the other side then a same atom or, similar part of the molecule will obtain. If it is present in a molecule, the molecule will become optically inactive in spite of the chiral centre present in the molecule.
Example - Tetrafluoro ethene contains i point.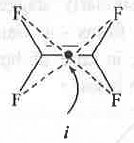
- Alternating Axis of Symmetry (Sn): A molecule is said to have alternating axis of symmetry of n fold if the molecule is rotated through an angle of 360°/n about the axis followed by reflection in a plane perpendicular to this axis results indistinguishable structure with the original one. Rotation followed by reflection or, reflection followed by rotation leads to the same results. If it is present in a molecule, the molecule will become optically inactive in spite of the chiral centre present in the molecule.
Example - ∝ -Truxillic acid contains S2 axis.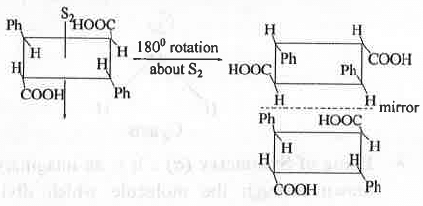
- External Compensation : When a molecule is present as equal mixture of enantiomers, it is called racemic mixture. The racemic mixture is optically inactive due to external compensation. Here, the dextrorotation of one enantiomer is compensated by the laevorotation of the other enantiomer.
4. R/S Nomenclature:
- First assign priority of the groups as 1, 2, 3 and 4 on the basis of C.I.P. (Cahn-Ingold-Prelog) rule.
- C.I.P. Rule : Higher is the atomic no. of the directly attached atom, higher will be its priority.
- B. If the 4-th group is at vertical position : 1 → 2 → 3 ⇛ Clockwise rotation ⇛ R and 1 → 2 → 3 ⇛ Anti-clockwise rotation ⇛ S
- If the 4-th group is at horizontal position : 1 → 2 → 3 ⇛ Clockwise rotation ⇛ S and 1 → 2 → 3 Anti-clockwise rotation ⇛ R
5. Conformational Isomerism :
A molecule can have two conformational isomers -
1. Eclipsed and 2. Staggered. Also, eclipsed conformer is sub-divided into two forms - a. Fully-eclipsed and b. Partly-eclipsed. Similarly, staggered conformer is subdivide! into two forms - a. Gauche and b. Anti. The four coi.fonners, in case of butane (for C2-C3 bond rotation) is shown below: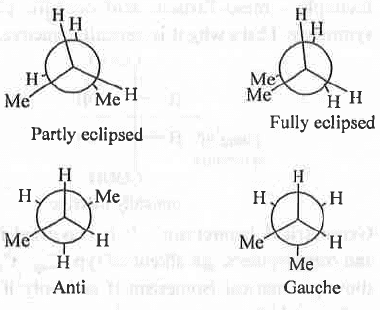
6. Stability of Conformers:
- General Cases : As fully eclipsed conformer involves highest steric repulsion between the two bulky -Me groups, it is least stable. Then, partly eclipsed conformer is also destabilised by the one bulky -Me and one small -H atom. Hence, it is somewhat more stable than the fully eclipsed conformer. Gauche conformer contains the two bulky -Me groups at an angle of 60°. Hence, it will have much less steric repulsion between the bulky - Me groups. Hence, it is much more stable than the both fully and partly eclipsed conformers. Finally, in case of anti conformer the two bulky -Me groups are 180° apart from each other. Hence, it involves least steric repulsion between the bulky -Me groups making it most stable. Hence, the order of stability of the conformers : Anti > Gauche > Partly eclipsed > Fully eclipsed.
- Exceptional Cases : In general, due to less crowding anti conformer is more stable than the gauche conformer. But, in some cases gauche conformer become more stable than the anti conformer. This is due to the following reasons:
- Formation of intra-molecular H-bonding - Ethylene glycol.
- Hyperconjugation - 1,2-Difluoro ethane
- Electrostatic interaction - 1 -Bromopropane

|
39 videos|92 docs|46 tests
|
FAQs on Revision Notes: Stereochemistry of Organic Molecules - Organic Chemistry
| 1. What is stereochemistry in organic molecules? |  |
| 2. Why is stereochemistry important in organic chemistry? |  |
| 3. What are enantiomers and how are they related to stereochemistry? |  |
| 4. How can stereochemistry be determined experimentally? |  |
| 5. What are some applications of stereochemistry in the pharmaceutical industry? |  |

















