Important S-Block Elements & Their Compounds Formulas for JEE and NEET
Group 1 of the periodic table consists of the elements : lithium, sodium, potassium, rubidium, caesium and francium. The elements of Group 2 include beryllium, magnesium, calcium, strontium, barium and radium.
Hydration Enthalpy : The hydration enthalpies of alkali metal ions decrease with increase in ionic sizes. Li+ has maximum degree of hydration and for this reasons lithium salts are mostly hydrated e.g., LiCI . 2H2O
Physical properties : All the alkali metal are silvery white, soft and light metals. Because of the larger size, these element have low density. The melting and boiling point of the alkali metals are low indicating weak metallic bonding alkali metals and their salts impart characteristic colour to an oxidizing flame.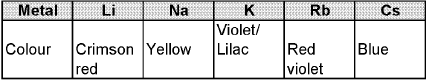
Chemical Properties: The alkali metal are highly reactive due to their larger size and low ionization enthalpy.
- Reactivity towards air : They burn vigorously in oxygen forming oxides. Lithium forms monoxide, sodium forms peroxide, the other metals form superoxide.
- Reducing nature: The alkali metals, are strong reducing agents, lithium being the most and sodium the least powerful.
- Solution in liquid ammonia: The alkali metals dissolve in liquid ammonia giving deep blue solution which are conducting in nature.
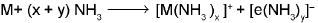 The blue colour of the solution is due to the ammoniated electron and the solutions is paramagnetic.
The blue colour of the solution is due to the ammoniated electron and the solutions is paramagnetic.
In concentrated solution, the blue colour changes to bronze colour and becomes, diamagnetic.
ANOMALOUS PROPERTIES OF LITHIUM
(i) exceptionally small size of its atom and ion, and (ii) high polarising power (i.e., charge/ radius ratio). The similarity between lithium and magnesium is particularly striking and arises because of their similar size : atomic radii, Li = 152 pm, Mg = 160 pm; ionic radii: Li+ = 76 pm, Mg2+ = 72 pm.
GROUP 2 ELEMENTS : ALKALINE EARTH METALS
The first element beryllium differs from the rest of the member and shows diagonal relationship to aluminium.
Hydration Enthalpies
Hydration enthalpies of alkaline earth metal ions. Be2+ > Mg2+ > Ca2+ > Sr2+ > Ba2+. The hydration enthalpies of alkaline earth metal ions are larger than those of alkali metal ions. Thus, compounds of alkaline earth metals are more extensively hydrated than those of alkali metals , e.g., MgCI2 and CaCI2 exist as MgCI2.6H2O and CaCI2. 6H2O while NaCI and KCI do not form such hydrates.
Physical Properties
The alkaline earth metals, in general, are silvery white, lustrous and relatively soft but harder than the alkali metals. The melting and boiling point of these metals are higher due to smaller sizes. Because of the low ionisation enthalpies they are strongly electropositive in nature. The electrons in beryllium and magnesium are too strongly bound to get excited by flame. Hence these elements do not impart any colour to the flame. Calcium, strontium and barium impart characteristic colour to the flame.
Chemical Properties
- Reactivity towards air and water: Beryllium and magnesium are inert to oxygen and water. Magnesium is more electropositive and burns with dazzling brilliance in air to give MgO and Mg3N2 .Calcium, strontium and barium are readily attacked by air to form the oxide and nitride.
- Reducing nature : The alkaline earth metals are strong reducing agent. This is indicated by large negative value of their reduction potentials.
- Solution in liquid ammonia: The alkaline earth metals dissolve in liquid ammonia to give deep blue black solution forming ammoniated ions.
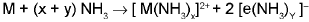 From these solutions, the ammoniates, [M(NH3)6]2+ can be recovered.
From these solutions, the ammoniates, [M(NH3)6]2+ can be recovered.
ANOMALOUS BEHAVIOUR OF BERYLLIUM
Beryllium the first member of the Group 2 metals, shows anomalous behaviour as compared to magnesium and rest of the members. Further, it shows diagonal relationship to aluminium.
Diagonal Relationship between Beryllium and Aluminium
The ionic radius of Be2+ is estimated to be 31 pm; the charge/radius ratio is nearly the same as that of the Al3+ ion. Hence beryllium resembles aluminium in some ways.
Compounds of s-block elements :
- Sodium Oxide (Na2O) :
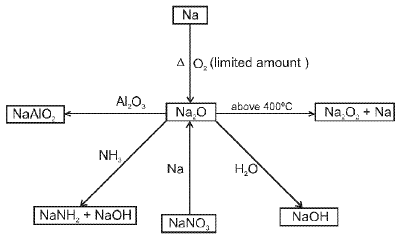
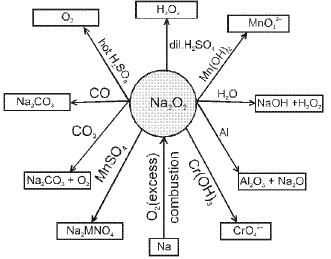
- Sodium peroxide (Na2O2) :
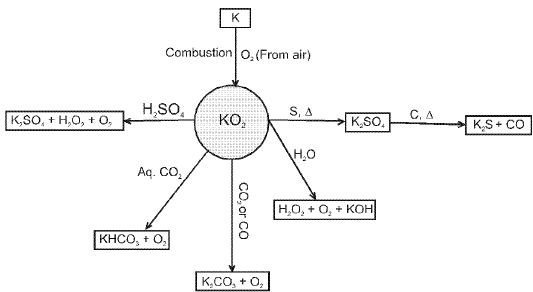
- Potassium Super oxide (KO2) :
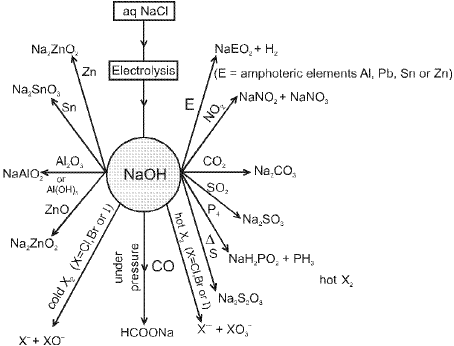
- Sodium Hydroxide (NaOH):
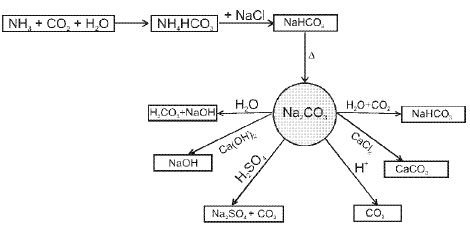
- Sodium Carbonate (Na2CO3) :
- Potassium sulphate (K2SO4) :
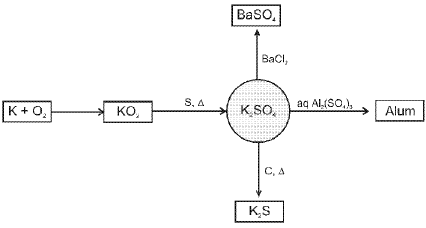
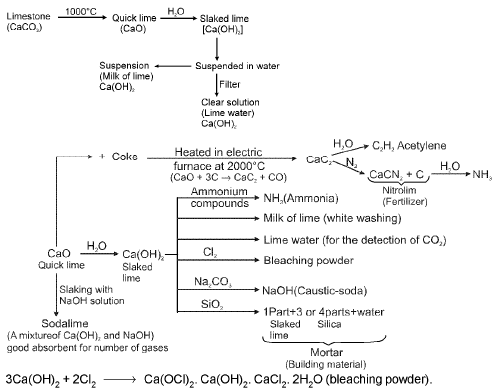
- Quick Lime, Slaked Lime and Lime Water :
|
75 videos|278 docs|78 tests
|





















