NEET Exam > NEET Notes > Chemistry Class 11 > Mindmap: Classification of Elements & Periodicity in Properties
Mindmap: Classification of Elements & Periodicity in Properties | Chemistry Class 11 - NEET PDF Download
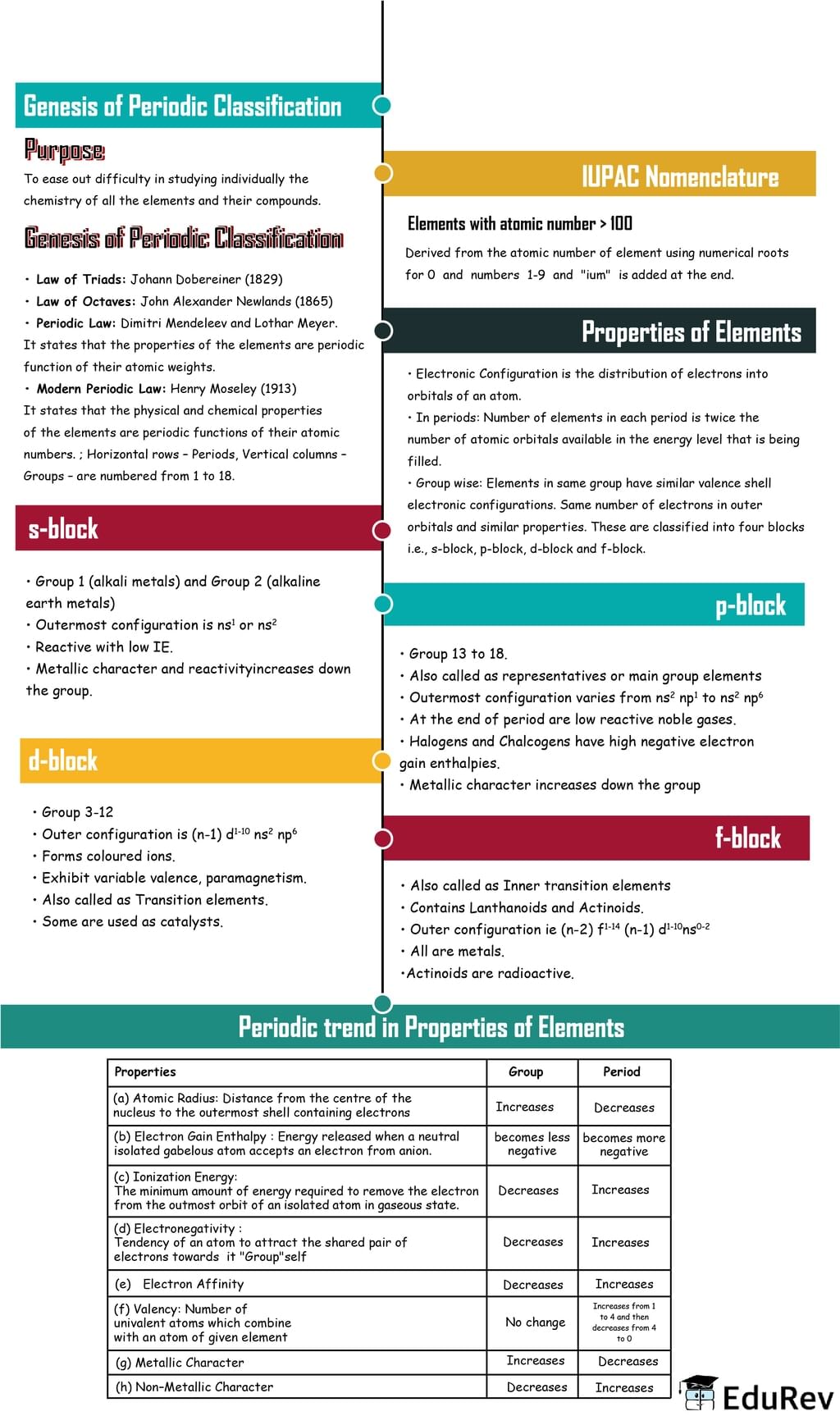
The document Mindmap: Classification of Elements & Periodicity in Properties | Chemistry Class 11 - NEET is a part of the NEET Course Chemistry Class 11.
All you need of NEET at this link: NEET
|
127 videos|244 docs|87 tests
|
FAQs on Mindmap: Classification of Elements & Periodicity in Properties - Chemistry Class 11 - NEET
| 1. What is the periodic table and why is it important? |  |
Ans. The periodic table is a tabular arrangement of chemical elements, organized based on their atomic numbers, electron configurations, and recurring chemical properties. It is important because it provides a systematic way to understand and predict the behavior of elements, their atomic structures, and their relationships with other elements.
| 2. How are elements classified in the periodic table? |  |
Ans. Elements in the periodic table are classified into groups and periods. Groups represent columns and share similar properties due to the same number of valence electrons, while periods represent rows and indicate the energy level of the outermost shell. The classification is based on the electronic configuration and recurring chemical properties.
| 3. What are the trends in periodicity of properties across a period? |  |
Ans. Across a period, the general trends in periodicity of properties include an increase in the number of protons, electrons, and atomic mass, as well as a decrease in atomic radius. Additionally, there is a gradual shift from metallic to non-metallic character, increasing electronegativity, and decreasing reactivity with metals becoming less reactive and non-metals becoming more reactive.
| 4. How do elements in the same group show similar properties? |  |
Ans. Elements in the same group share similar properties because they have the same number of valence electrons, which determines the chemical behavior of an element. The valence electrons are located in the outermost energy level, and elements with the same number of valence electrons tend to have similar reactivity, chemical bonding, and physical properties.
| 5. What is the significance of the periodic law in understanding element properties? |  |
Ans. The periodic law states that the properties of elements are periodic functions of their atomic numbers. This law provides a fundamental basis for understanding and predicting the behavior of elements. It allows scientists to organize elements in a meaningful and systematic way, revealing trends and patterns in their properties, such as atomic radius, electronegativity, ionization energy, and more.

|
Explore Courses for NEET exam
|

|
Signup for Free!
Signup to see your scores go up within 7 days! Learn & Practice with 1000+ FREE Notes, Videos & Tests.
Related Searches


















