Important Qualitative Analysis Formulas for JEE and NEET
Charcoal Cavity Test: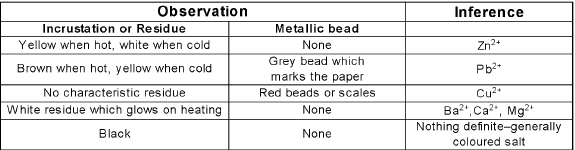
Cobalt Nitrate Test: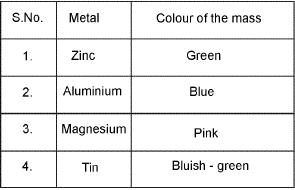
Flame test :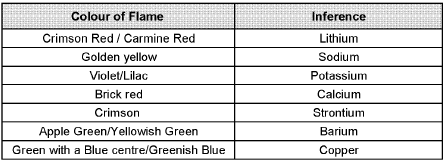
Borax Bead test: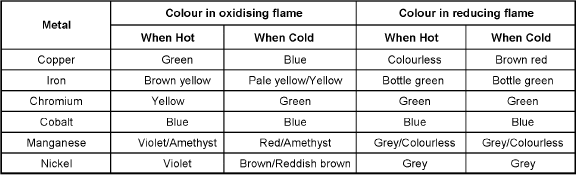
Analysis of ANIONS (Acidic Radicals) :
(a) DILUTE SULPHURIC ACID/DILUTE HYDROCHLORIC ACID GROUP :
1. CARBONATE ION (CO32-) :
- Dilute H2SO4 test : A colourless odourless gas is evolved with brisk effervescence.
CaCO3 + H2SO4 → CaSO4 + H2O + CO2↑ - Lime water/Baryta water (Ba(OH)2) test:
CO2 + Ca(OH)2 → CaCO3↓, (milky) + H2O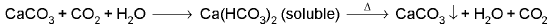
2. SULPHITE ION ( SO32-) :
- Dilute H2SO4 test :
CaSO3 + H2SO4 → CaSO4 + H2O + SO2↑ ; SO2 has suffocating odour of burning sulphur. - Acidified potassium dichromate test : The filter paper dipped in acidified K2Cr2O7 turns green.
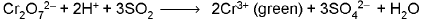
- Barium chloride/Strontium chloride solution :
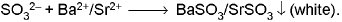
White precipitate dissolves in dilute HCI. BaSO3 ↓ + 2H+ → Ba2+ + SO2 ↑ + H2O.
3. SULPHIDE ION (S2-):
- Dilute H2SO4 test : Pungent smelling gas like that of rotten egg is obtained.
S2- + 2H+ → H2S↑ - Lead acetate test : (CH3COO)2Pb + H2S → PbS↓ (black) + 2CH3COOH.
- Sodium nitroprusside test: Purple coloration is obtained.
S2- + [Fe(CN)5 (NO)]2- → [Fe(CN)5NOS]4- (violet). - Cadmium carbonate suspension/ Cadmium acetate solution :
Na2S + CdCO3 → CdS↓ (Yellow) + Na2CO3
4. NITRITE ION ( NO2-):
- Dilute H2SO4 test :
NO2- + H+ → HNO2 ; (2 HNO2 → H2O + N2O3);
3HNO2 → HNO3 + 2NO + H2O ; 2NO + O2 → 2NO2 ↑ - Starch iodide test:
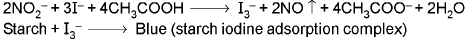
5. ACETATE ION (CH3COO-)
- Dilute H2SO4 test : (CH3COO)2Ca + H2SO4 → 2CH3COOH (vinegar like smell) + CaSO4
- Neutral ferric chloride test :
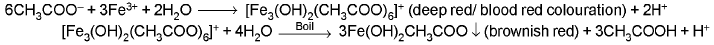
(b) CONC. H2SO4 GROUP :
1. CHLORIDE ION (Cl-):
- Concentrated H2SO4 test : Cl- + H2SO4 → HCI (colourless pungent smelling gas) + HSO4-
- NH4OH + HCI → NH4CI ↑ (white fumes) + H2O.
- Silver nitrate test : Cl- + Ag+ → AgCI↓ (white)
White precipitate is soluble in aqueous ammonia and precipitate reappears with HNO3.
AgCI + 2NH4OH → [ Ag(NH3)2]CI (Soluble) + 2H2O ; [Ag(NH3)2]CI + 2H+ → AgCI↓ + 2NH4+.
- Chromyl chloride test:
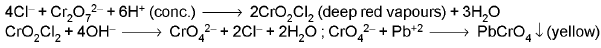
2. BROMIDE ION (Br-) : - Concentrated H2SO4 test :
2NaBr + H2SO4 → Na2SO4 + 2HBr ; 2HBr + H2SO4→ Br2 ↑ (reddish-brown) + 2H2O + SO2 - Silver nitrate test : NaBr + AgNO3 → AgBr↓ (pale yellow) + NaNO3
Yellow precipitate is partially soluble in dilute aqueous ammonia but readily dissolves in concentrated ammonia solution. AgBr + 2NH4OH → [Ag(NH3)2] Br + H2O
- Chlorine water test (organic layer test):
2Br- + Cl2 → 2CI- + Br2 ↑.
Br2 + CHCI3 / CCI4 → Br2 dissolve to give reddish brown colour in organic layer.
3. IODIDE ION (I-) : - Concentrated H2SO4 test : 2Nal + H2SO4 → Na2SO4 + 2HI
2HI + H2SO4 → l2 ↑ (pungent smelling dark violet) + 2H2O + SO2 - Starch paper test: Iodides are readily oxidised in acid solution to free iodine; the free iodine may than be identified by deep blue colouration produced with starch solution.
3I- + 2NO2- + 4H+ → l3- + 2NO ↑ + 2H2O. - Silver nitrate test : Bright yellow precipitate is formed.
I- + Ag+ → Agl↓
Bright yellow precipitate is insoluble in dilute aqueous ammonia but is partially soluble in concentrated ammonia solution.
- Chlorine water test (organic layer test):
2Nal + Cl2 → 2NaCI + l2
l2 + CHCI3 → l2 dissolves to give violet colour in organic layer.
4. NITRATE ION (NO3-) : - Concentrated H2SO4 test: Pungent smelling reddish brown vapours are evolved.
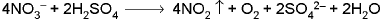
Addition of bright copper turnings or paper pellets intensifies the evolution of reddish brown gas.
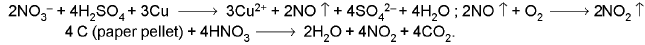
- Brown ring test:
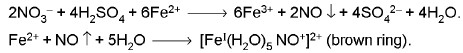
3. Miscellaneous Group :
1. SULPHATE ION (SO42-) :
- Barium chloride test : Na2SO4 + BaCI2 → BaSO4↓ (white) + 2NaCI.
White precipitate is insoluble in warm dil. HNO3 as well as HCI but moderately soluble in boiling concentrated hydrochloric acid.
- Lead acetate test : Na2SO4 + (CH3COO)2Pb → PbSO4 ↓ (White) + 2CH3COONa
White precipitate soluble in excess of hot ammonium acetate.
PbSO4 + 2CH3COONH4 → (CH3COO)2Pb (soluble) + (NH4)2SO4
2. PHOSPHATE ION (PO43-):
- Ammonium molybdate test:
Na2 HPO4 (aq) + 12(NH4)2MoO4 + 23HNO3 → (NH4)3PMo12O40↓ (canary yellow) + 2NaNO3 + 21NH4NO3 + 12H2O
ANALYSIS OF CATIONS
1. AMMONIUM ION (NH4+) :
2NH3 + Mn2+ + H2O2 + H2O → MnO(OH)2 ↓ (brown) + 2NH4+
Nessler's reagent (Alkaline solution of potassium tetraidomercurate(ll):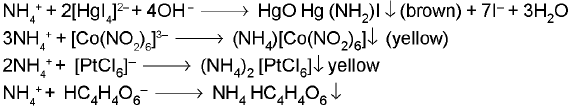
Ist GROUP (Pb2+, Hg22+, Ag+):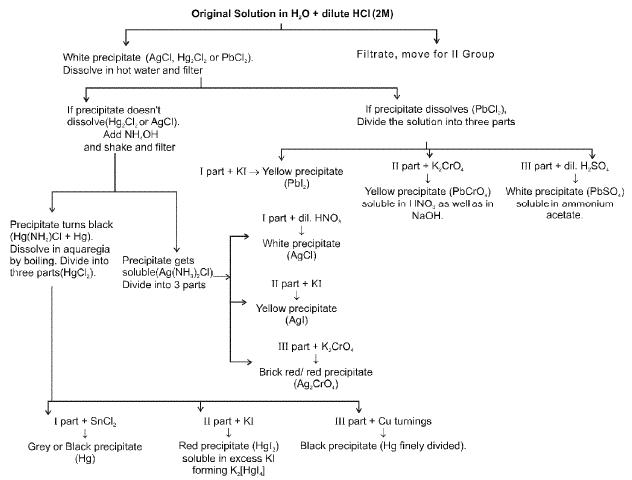
IIA Group (Hg2+, Pb2+, Bi3+, Cu2+, Cd2+)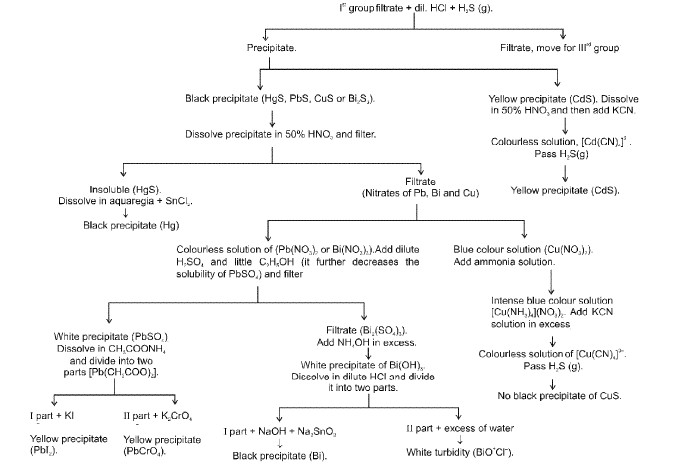
IIB Group (As3+, Sb3+, Sn2+, Sn4+)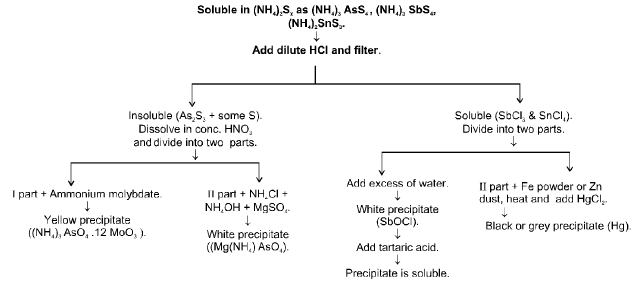
IIIrd Group (Al+3, Cr+3, Fe+3)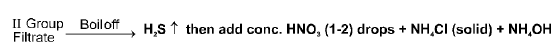
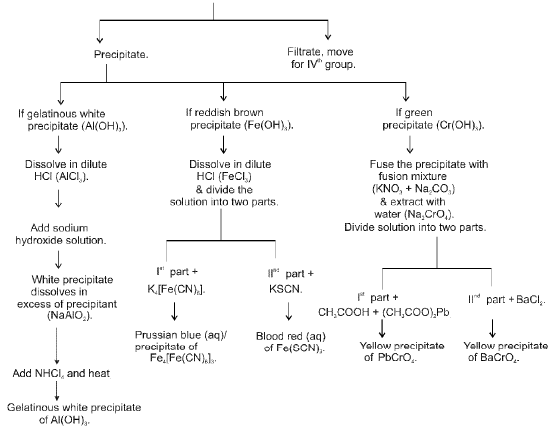
IVth GROUP (Zn2+, Mn2+, Ni2+, Co2+)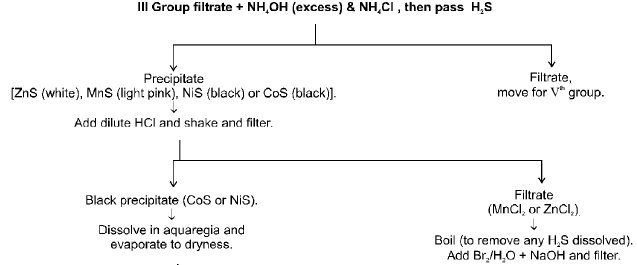
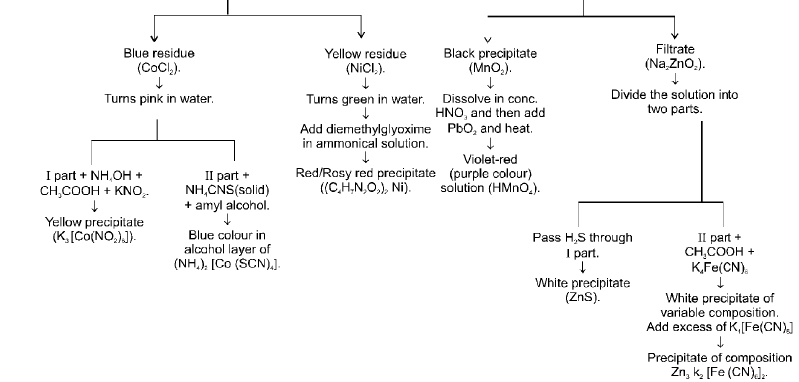
Vth Group (Ba2+, Sr2+, Ca2+) :
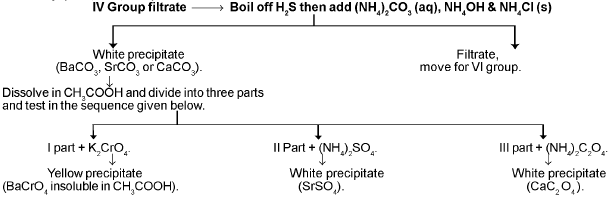
VIth GROUP :
MAGNESIUM ION (Mg2+) :
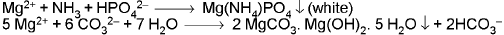
Titan Yelllow (a water soluble yellow dyestuff): It is adsorbed by Mg(OH)2 producing a deep red colour or precipitate.
|
75 videos|278 docs|78 tests
|
FAQs on Important Qualitative Analysis Formulas for JEE and NEET
| 1. What is qualitative analysis in chemistry? |  |
| 2. What are the main methods used in qualitative analysis? |  |
| 3. How do you perform a flame test, and what does it indicate? |  |
| 4. What role do indicators play in qualitative analysis? |  |
| 5. How can qualitative analysis be applied in real-world scenarios? |  |





















