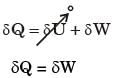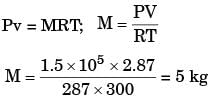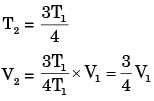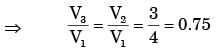GATE Past Year Questions: Thermodynamic System & Processes | Thermodynamics - Mechanical Engineering PDF Download
Q1: Consider a mixture of two ideal gases, X and Y, with molar masses 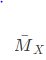 =10kg/and
=10kg/and 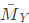 =20kg/kmol, respectively, in a container. The total pressure in the container is 100 kPa, the total volume of the container is 10m3and the temperature of the contents of the container is 300 K. If the mass of gas-X in the container is 2 kg, then the mass of gas-Y in the container is ____ kg. (Rounded off to one decimal place) Assume that the universal gas constant is 8314 J K mol -1K1- (2023)
=20kg/kmol, respectively, in a container. The total pressure in the container is 100 kPa, the total volume of the container is 10m3and the temperature of the contents of the container is 300 K. If the mass of gas-X in the container is 2 kg, then the mass of gas-Y in the container is ____ kg. (Rounded off to one decimal place) Assume that the universal gas constant is 8314 J K mol -1K1- (2023)
(a) 2
(b) 4
(c) 6
(d) 8
Ans: (b)
Sol: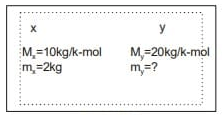
By gas law
Pv=nRT
where n= total value of gas in container
100x10=n x8.314x300
n=0.4
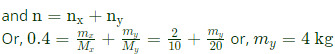
Q2: Which one of the following statements is FALSE?
(a) For an ideal gas, the enthalpy is independent of pressure.
(b) For a real gas going through an adiabatic reversible process, the process equation is given by PVγ = constant, where P is the pressure, V is the volume and γ is the ratio of the specific heats of the gas at constant pressure and constant volume.
(c) For an ideal gas undergoing a reversible polytropic process PV1.5= constant, the equation connecting the pressure, volume and temperature of the gas at any point along the process is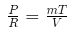 where R is the gas constant and m is the mass of the gas.
where R is the gas constant and m is the mass of the gas.
(d) Any real gas behaves as an ideal gas at sufficiently low pressure or sufficiently high temperature. (2023)
Ans: (b)
Sol:
Ideal gas follow the adiabatic equation P V γ =C and also follow the gas law P V = m R T PV=mRT
Any real gas at low pressure and high temperature follow gas law, e.g. Air conditioning system.Real gas follow Van der waal's gas equation for an adiabatic process as 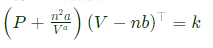
where
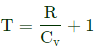
k= nRZ
Enthalpy of an ideal gas is independent of pressure at constant temperature and the internal energy of an ideal gas is independent of volume at constant temperature
Q3: At steady state, 500kg/s of steam enters a turbine with specific enthalpy equal to 3500kJ/kg and specific entropy equal to 6.5kj kg-1k1- expands reversibly in the turbine to the condenser pressure. Heat loss occurs reversibly in the turbine at a temperature of 500K. If the exit specific enthalpy and specific entropy are 2500kJ/kg and 6.3kj kg -1k1- respectively, the work output from the turbine is ________ MW (in integer). (2022 SET 2)
(a) 320
(b) 650
(c) 275
(d) 450
Ans:(d)
Sol: Mass flow rate of stem m ˙=500kg/s 
Surrounding temperature To=500K
Work output from turbine
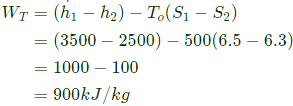
Power output of turbine 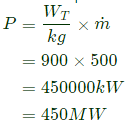
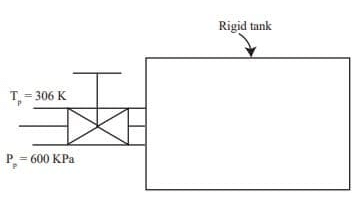
Q4: A rigid tank of volume of 8 m3 is being filled up with air from a pipeline connected through a valve. Initially the valve is closed and the tank is assumed to be completely evacuated. The air pressure and temperature inside the pipeline are maintained at 600 kPa and 306 K, respectively. The filling of the tank begins by opening the valve and the process ends when the tank pressure is equal to the pipeline pressure. During the filling process, heat loss to the surrounding is 1000 kJ. The specific heats of air at constant pressure and at constant volume are 1.005 kJ/kg .K and 0.718 kJ/kg. K, respectively. Neglect changes in kinetic energy and potential energy.
The final temperature of the tank after the completion of the filling process is _________ K (round off to the nearest integer). (2022 SET 2)
(a) 395
(b) 254
(c) 355
(d) 125
Ans: (a)
Sol:
 l:
l:
Initially the tank is completely evacuated (m1=0)
After the gas is filled with tank, the gas pressure in the tank is 600 kPa.
Heat lost to surroundings (Q) = 1000 kJ 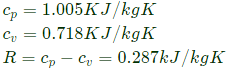
(Changes in KE and PE are negligible)
Final temperature of gas T f=?
m = mass of gas entering to tank
The energy balance equation is energy carried by gas in the pipe = energy of gas in the rigid tank + Heat lost to surrounding 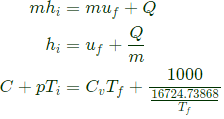
Mass of gas in the tank
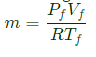
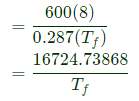
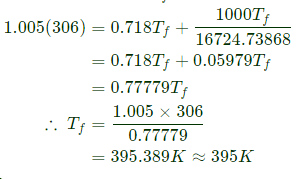
Q5: Which one of the following is an intensive property of a thermodynamic system? (2022 SET 2)
(a) Mass
(b) Density
(c) Energy
(d) Volume
Ans: (b)
Sol:
Intensive property -> Density
Mass, energy and volume are extensive properties.
Q6: Consider adiabatic flow of air through a duct. At a given point in the duct, velocity of air is 300 m/s, temperature is 330 K and pressure is 180 kPa. Assume that the air behaves as a perfect gas with constant cp=1.005 kJ/kg.K. The stagnation temperature at this point is ______K (round off to two decimal places). (2021 SET2)
(a) 374.71
(b) 352.24
(c) 874.65
(d) 458.32
Ans: (a)
Sol:
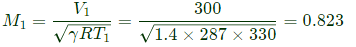
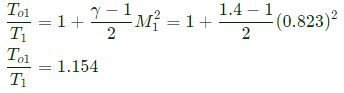
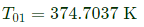
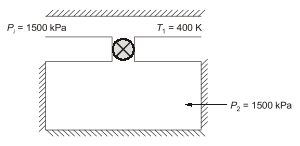
Q7: A rigid insulated tank is initially evacuated. It is connected through a valve to a supply line that carries air at a constant pressure and temperature of 250 kPa and 400 K respectively. Now the valve is opened and air is allowed to flow into the tank until the pressure inside the tank reaches to 250 kPa at which point the valve is closed. Assume that the air behaves as a perfect gas with constant properties cp =1.005kJ/kg. K, cv=0.718kJ/kg. K, R=0.287kJ/kg. K). Final temperature of the air inside the tank is _______K (round off to one decimal place).
(a) 512
(b) 248
(c) 688
(d) 560
Ans: (d)
Sol:
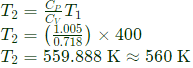
Q8 : A gas is heated in a duct as it flows over a resistance heater. Consider a 101 kW electric heating system. The gas enters the heating section of the duct at 100 kPa and 270C with a volume flow rate of 15 m3/s If heat is lost from the gas in the duct to the surroundings at a rate of 51 kW, the exit temperature of the gas is(Assume constant pressure, ideal gas, negligible change in kinetic and potential energies and constant specific heat; Cp=1 kJ/kg .K; R=0.5 kJ/kg .K)
(a) 320C
(b) 370C
(c) 53 0C
(d) 76 0C
Ans: (b)
Sol:
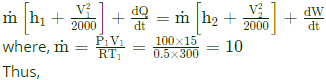
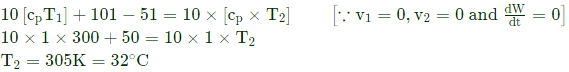
Q9: During a non-flow thermodynamic process(1-2) executed by a perfect gas, the heat interaction is equal to the work interaction Q1-2 W1-2) when the process is (2019 SET 1)
(a) Isentropic
(b) Polytropic
(c) Isothermal
(d) Adiabatic
Ans: (c)
Sol:
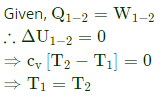
So, the process is isothermal.
Q10: The volume and temperature of air (assumed to be an ideal gas) in a closed vessel is 2.87 m3 and 300K, respectively. The gauge pressure indicated by a manometer fitted to the wall of the vessel is 0.5bar. If the gas constant of air is R=287J/kg ⋅K and the atmospheric pressure is 1 bar, the mass of air (in kg) in the vessel is (2017 SET2)
(a) 1.67
(b) 3.33
(d) 5.04
(c) 6.66
Ans: (c)
Sol: Pg =5bar=50kPa
R=287J/Kg=0.287kj/kg k
Ideas gas equation:
PV= mRT
Here P is absolute pressure:
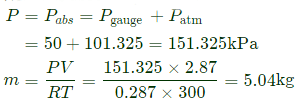
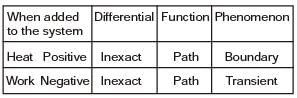
(i) They are boundary phenomena
(ii) They are exact differentials
(iii) They are path functions
[2016, Set-1]
[2011]
[2006]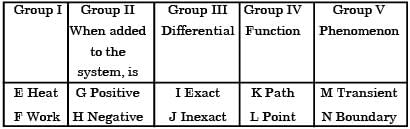
[1996]
[1994]
[1993]
[2019 , Set -1]
[2017, Set-2]
[2014, Set-3]
[2007]
The above cycle is represented on T-s plane by [2007]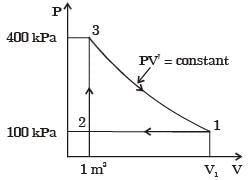
[2005]
(i) there must be one isothermal process,
(ii) there must be one isentropic process,
(iii) the maximum and minimum cycle pressures and the clearance volume are fixed, and
(iv) polytropic processes are not allowed. Then the number of possible cycles are
According to the first law of thermodynamics, equal areas are enclosed by [2005]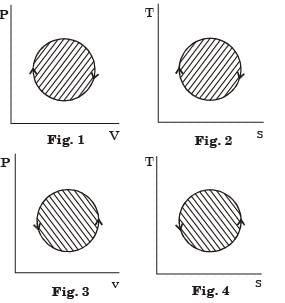
[1999]
|
29 videos|152 docs|36 tests
|
FAQs on GATE Past Year Questions: Thermodynamic System & Processes - Thermodynamics - Mechanical Engineering
| 1. What is a thermodynamic system? |  |
| 2. What are the different types of thermodynamic processes? |  |
| 3. How is work calculated in thermodynamic processes? |  |
| 4. What is the first law of thermodynamics? |  |
| 5. How do thermodynamic processes affect the efficiency of a system? |  |