GATE Past Year Questions: Availability & Irreversibility | Thermodynamics - Mechanical Engineering PDF Download
Q1: The Clausius inequality holds good for [GATE ME 2022, SET-1]
(a) any process
(b) any cycle
(c) only reversible process
(d) only reversible cycle
Ans: (b)
The Clausius inequality holds good for any cycle.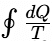 = 0 ⇒ Reversible cycle
= 0 ⇒ Reversible cycle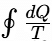 < 0⇒ Irreversible cycle
< 0⇒ Irreversible cycle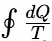 >0⇒ Impossible cycle
>0⇒ Impossible cycle
Q1: Keeping all other parameters identical, the Compression Ratio (CR) of an air standard diesel cycle is increased from 15 to 21. Take ratio of specific heats = 1.3 and cut-off ratio of the cycle rc = 2
The difference between the new and the old efficiency values, in percentage,
(ηnew|CR = 21) - (ηold |CR = 15) = _______ %. (round off to one decimal place) [GATE ME 2020, SET-2]
Ans: (4.6 to 4.9)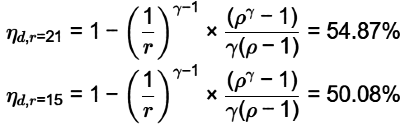
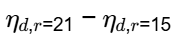 = 4.8%
= 4.8%
[1995]
[2000]
[2003]
[2004]
[2013]
[2014]
|
29 videos|150 docs|36 tests
|
FAQs on GATE Past Year Questions: Availability & Irreversibility - Thermodynamics - Mechanical Engineering
| 1. What is the concept of availability in thermodynamics? |  |
| 2. How is irreversibility defined in mechanical engineering? |  |
| 3. What are the main factors that contribute to irreversibility in thermodynamic processes? |  |
| 4. How can the concept of availability be applied in engineering design? |  |
| 5. What is the relationship between availability and entropy? |  |