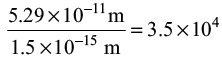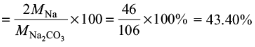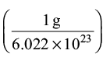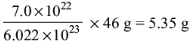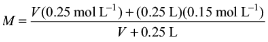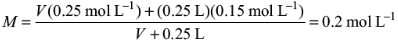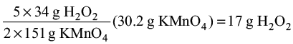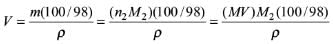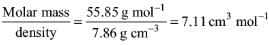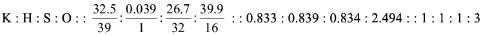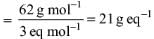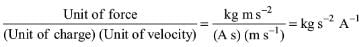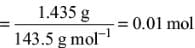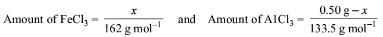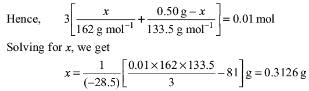Basic Concepts of Chemistry
1. The value of Avogadro constant is
(a) 6.022 x 1022 atoms
(b) 6.022 x 1023 atoms
(c) 6.022 x 1023 mol–1
(d) 6.022 x 1022 mol–1
Ans: c
Solution: Avogadro constant is 6.022 x 1023 mol–1.
2. The number of significant digits in the number 126000 is
(a) 3
(b) 4
(c) 5
(d) 6
Ans: a
Solution: There are 3 digits. The last three zeros are not significant.
3. The relative atomic mass of sodium is 23. Which of the following statements is not correct about sodium?
(a) Atomic mass of sodium is 23 u.
(b) Atomic mass of sodium is 3.82 x 10-26 kg.
(c) Molar mass of sodium is 23 g mol-1.
(d) The number of atoms in 24 kg of sodium is 6.022 x 1023.
Ans: d
Solution: The number of atoms in 23 g of Na will be equal to 6.022 x 1023
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:Law of conservation of masses was firstly established by
Explanation
Law of conservation of mass was established by Lavoisier.
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The atomic mass unit is equal to
Explanation
1 a.m.u = 1.66 x 10–27 kg.
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:Which of the following expressions is dimensionally correct?
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:Which of the concentration units is temperature dependent?
Explanation
Molarity is dependent on temperature.
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:One femtometer stands for
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The unit of ‘amount of substance’ is
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:Which one of the following is the correct conversion expression of 1 J?
Explanation
Report a problem
4. The radius of a hydrogen atom is 5.29 X 10-11 m and that of a proton is 1.5 x10-15 m. The ratio of the radius of atom to the radius of proton in the scientific notation will be
(a) 3.526 x 104
(b) 35.266 x 103
(c) 3.5 x 104
(d) 3.526 6667 x103
Ans: c
Solution: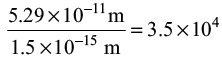
5. Mass percent of Na in Na2CO3 is
(a) 21.52%
(b) 31.20%
(c) 38.20%
(d) 43.40%
Ans: d
Solution: Mass percent of Na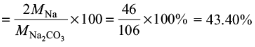
6. Select the quantity of NO2 with highest mass:
(a) 100 amu
(b) 1.0 x 10–3 g
(c) 7.0 x 1022 molecules
(d) 8.0 x 10–1 mol
Ans: d
Solution: 100 amu = (100)
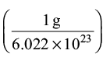
= 1.66 x 10–22 g
Mass of 7.0 x 1022 molecules =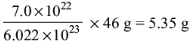
Mass of 8.0 x 10–1 mol = 0.8 x 46 g = 36.8 g
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The volume of concentrated sulphuric acid (98 mass % H2SO4, density 1.84 g cm–3) required to prepare 5 dm3 of 0.5 mol dm–3 solution of sulphuric acid is
Explanation
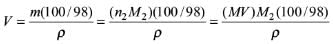
= {(0.5 mol dm–3) (5 dm3) (98 g mol–1) (100/98)}/(1.84 g cm–3) = 136 cm3
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:Iron has a density of 7.86 g cm–3 and an atomic mass of 55.85 amu. The volume occupied by 1 mol of Fe is
Explanation
Molar volume = Molar Mass/density = 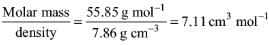
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The combustion of 4.24 mg of an organic compound produces 8.45 mg of CO2 and 3.46 mg of water. The mass percentages of C and H in the compound, respectively, are
Explanation
Mass percent of C = (12/44) (8.45/4.24)100 = 54.4%
Mass percent of H = (2/18) (3.46/4.24)100 = 9.1%
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The simplest formula of the compound containing 32.5% K, 0.839% H, 26.7% S and 39.9% O by mass is
Explanation
Ratio of atoms
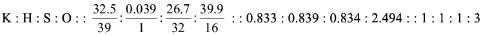
Empirical formula = KHSO3
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:If in a reaction HNO3 is reduced to NO, the mass of HNO3 absorbing one mole of electrons would be
Explanation
We have HNO3 → NO
Change in oxidation number = -3.
Equivalent mass of HNO3
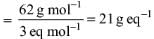
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The SI unit of magnetic flux density is
Explanation
By definition, F = qBv. Hence,
Unit of B = 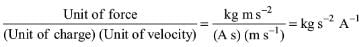
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:Boron occurs in two varieties, namely, 10B (atomic mass: 10.01 amu) 11B (atomic mass: 11.01 amu). The atomic mass of the naturally occurring element is reported as 10.82 amu. The percent of 10B in this naturally occurring boron is
Explanation
If is the fraction of 10B, we have (10.01 amu) + (1 – ) (11.01 amu) = 10.82 amu
This gives = 0.19. Hence, the percentage is 19%.
Report a problem
7. The volume of 0.25 M NaOH to be added to 250 mL of 0.15 M NaOH so that the resultant solution is 0.2 M would be
(a) 250 mL
(b) 350 mL
(c) 450 mL
(d) 550 mL
Ans: a
Solution: Let V be the volume of 0.25 M NaOH solution. Total amount of NaOH after mixing the two solutions is n = V (0.25 mol L–1) + (0.25 L) (0.15 mol L–1)
Total volume of the solution = V + 0.25 L
Molarity of the resultant solution
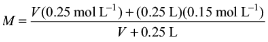
Equating this to 0.2 M, we get
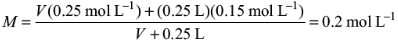
Solving for V, we get V = 0.25 L = 250 mL
8. The mass of H2O2 that is completely oxidised by 30.2 g of KMnO4 (molar mass = 158 g mol–1) in acidic medium is
(a) 12 g
(b) 15 g
(c) 17 g
(d) 1 g
Ans: c
Solution: The reaction is 2KMnO4 + 5H2O2 + 6H+ → 2Mn2+ + 5O2 + 8H2O + 2K+
2 x 151 g of KMnO4 reacts completely with 5 x 34 g of H2O2
30.2 g of KMnO4 reacts completely with 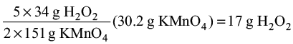
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The sulphur in 1.0 g sample of steel is burned to sulphur dioxide and absorbed in 50.0 mL of 0.1 M sodium hydroxide solution. The excess sodium hydroxide requires 24.0 mL of 0.15 M hydrochloric acid solution for neutralization. The percentage of sulphur in the sample is
Explanation
The reaction of NaOH and SO2 is 2NaOH + SO2 → Na2SO3 + H2O
Amount of NaOH to start with = VM = (50 x 10–3 L) (0.1 mol L–1) = 5 x 10–3 mol
Amount of NaOH after the absorption of SO2 = (24.0 x 10–3 L) (0.15 mol L–1) = 3.6 x 10–3 mol
Amount of NaOH used in absorbing SO2 = 5 x 10–3 mol – 3.6 x 10–3 mol = 1.4 x 10–3 mol
Amount of SO2 formed = (0.5) (1.4 x 10–3 mol) = 7.0 x 10–4 mol
Amount of S in the steel = 7.0 x 10–4 mol
Mass percent of S = 
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:A 0.50 g sample containing only anhydrous FeCl3 (molar mass: 162.5 g mol-1) and AlCl3 (molar mass: 133.5 g mol-1) yielded 1.435 g of AgCl (molar mass: 143.5 g mol-1). The mass of FeCl3 in the sample is
Explanation
Report a problem
Question for Solved Examples: Some Basic Concepts of Chemistry
Try yourself:The volume of water needs to be added to 10.0 mL of nitric acid (density: 1.40 g ml-1) containing 70 mass percent of acid to prepare 1.0 M solution would be
Explanation
Mass of 10.0 mL of nitric acid, m = Vρ = (10.0 mL)(1.40 g mL–1) = 14.0 g
Mass of nitric acid in this solution = (14.0 g) (70/100) = 9.8 g
Amount of nitric acid, n = m/M = 9.8 g/63 g mol–1 = 0.1556 mol
Molarity of solution
M = n/V = (0.1556 mol / 0.01L) = 15.56 M
This solution is to be diluted to prepare a 1.0 M solution.
Volume of the resultant solution
= (10mL)(15.56M) / (1.0M) = 155.6mL
Volume of water to be added = 155.6 mL – 10.0 mL = 145.6 mL
Report a problem